DICHOTOMOUS KEY AND BACTERIAL IDENTIFICATION DEMONSTRATION LAB
LEARNING OBJECTIVES
Demonstrate competency of microbiology laboratory techniques by separating and identifying microorganisms.
Design a dichotomous key to differentiate possible Gram-negative bacteria.
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation.
Apply various laboratory techniques to identify types of microorganisms.
Identify structural characteristics of the major groups of microorganisms.
Compare and contrast prokaryotic cell and eukaryotic cell.
Compare and contrast the physiology and biochemistry of the various groups of microorganisms.
constructing a DICHOTOMOUS KEY
A dichotomous key is a useful scientific tool used to identify organisms based on their characteristics. Dichotomous comes from the Greek “di” for “two” and “tome” for “cutting instrument.” The key consists of a series of paired statements about characteristics, providing a step wise guide toward identifying each organism. As the user proceeds from one step to the next, the clues gradually narrow down the list of possible organisms until all are identified. In a branching layout of a dichotomous key, each of an organism’s characteristics are laid out in a style similar to a flow chart. Each characteristic begins a new “branch” of the tree, with each subsequent question being a sub-branch. Identification is reached at the end of the tree’s “branches. “
Example of a branching dichotomous key
construct a dichotomous key
Use the information in the Test Results of Gram-Negative Bacteria in the table below to construct a branching dichotomous key. Your dichotomous key must include all the bacteria (8 total). Try to begin your key with a biochemical test that divides the bacteria in about half. Be efficient in your test choices. You may use a test as many times as you like. You cannot use unreliable test results to identify an organism.
Selective and Differential media and biochemical testS overview
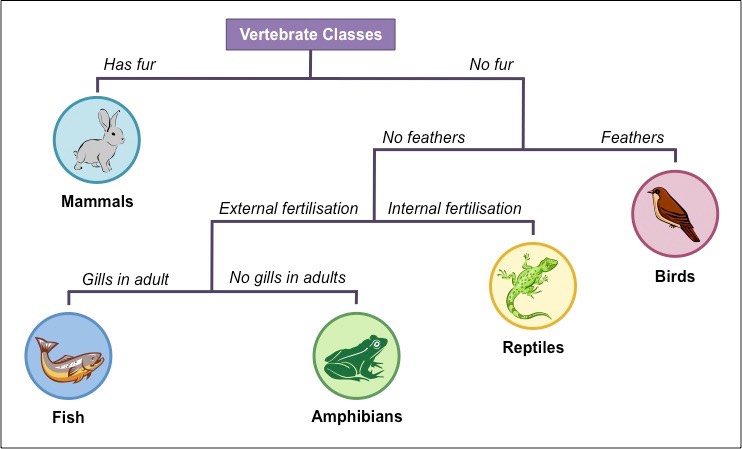
GLUCOSE FERMENTATION TEST
A carbohydrate fermentation broth uses a particular sugar, such as glucose or sucrose to determine if bacteria can ferment that sugar into acidic end products. A pH indicator will turn yellow if acid is produced.
AFTER INCUBATION
Yellow broth = Positive test
Red broth = Negative test

CITRATE TEST
This test uses Simmon’s citrate agar to determine if the organism can use citrate as its only carbon source. Since all organisms need carbon to survive, bacteria that cannot use the citrate will not grow. The agar contains citrate and ammonium ions (nitrogen source) and bromthymol blue as a pH indicator
AFTER INCUBATION
Bright blue = Positive
No color change (stays green) = Negative

INDOLE TEST
The Indole test uses tryptone broth to detect the organism’s ability to produce tryptophanase. This enzyme will split the amino acid tryptophan into indole and pyruvic acid. Indole forms a red ring with the addition of Kovac’s reagent.
AFTER INCUBATION add 10 drops of Kovac’s reagent to the tryptone tube
Red ring = Positive
No red ring = Negative
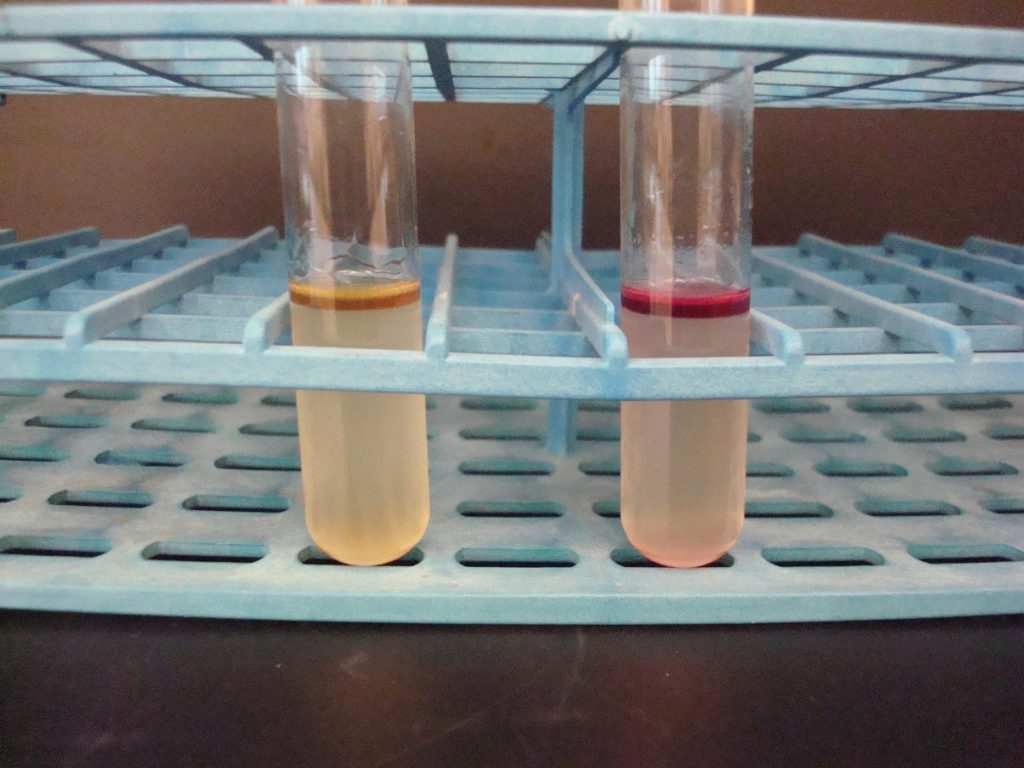
MOTILITY TEST
The motility test detects the ability of bacteria to produce flagella. (The media is a semi-solid agar with a colorless dye). As bacteria grow, the dye is reduced by the bacteria and turns red. Bacterial growth is detected by the presence of the red color. Observe the bacterial growth in the media. If the bacteria migrate away from the inoculation stab, the red dye will be diffused throughout the tube where the bacteria are growing. If the bacteria are not able to migrate, the red dye will be seen only in the stab line where the bacteria are growing.
AFTER INCUBATION
Diffused red color = Positive
Red color only at the stab line = Negative
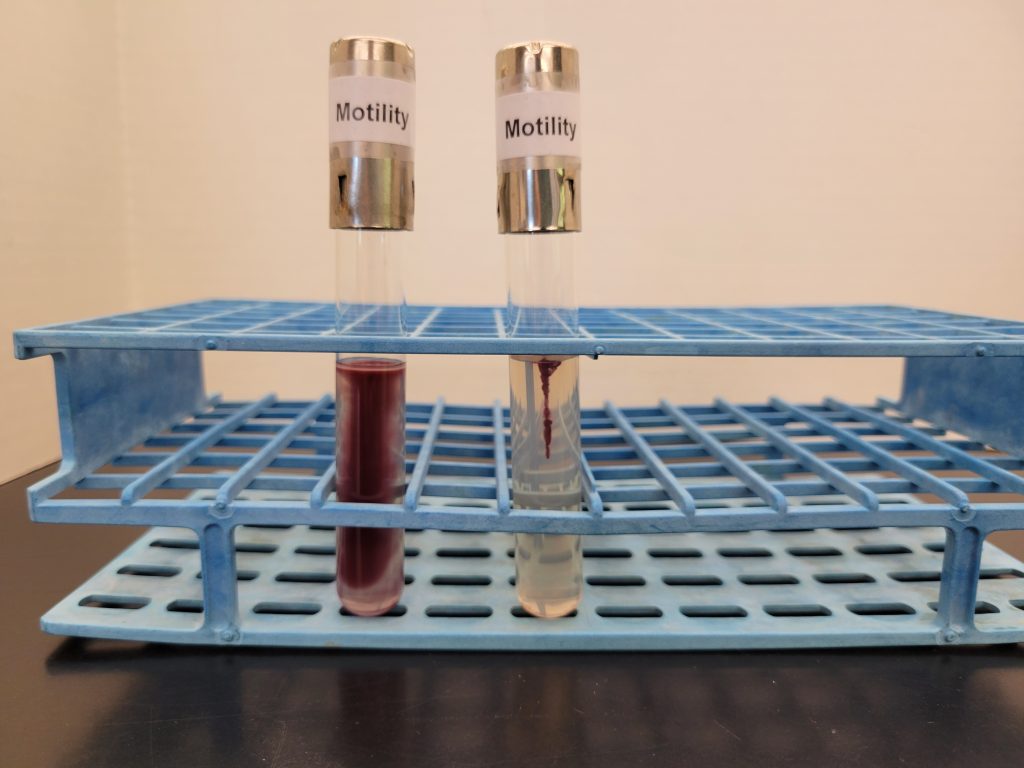
UREASE TEST
This test detects the ability of the bacteria to produce the enzyme urease. The media contains urea and the pH indicator, phenol red. Urease will hydrolyze urea to produce ammonia. The alkaline environment will change the phenol red indicator to hot pink. Observe the urea media for a color change.
AFTER INCUBATION
Hot pink media= Positive No color change (stays light salmon) = Negative
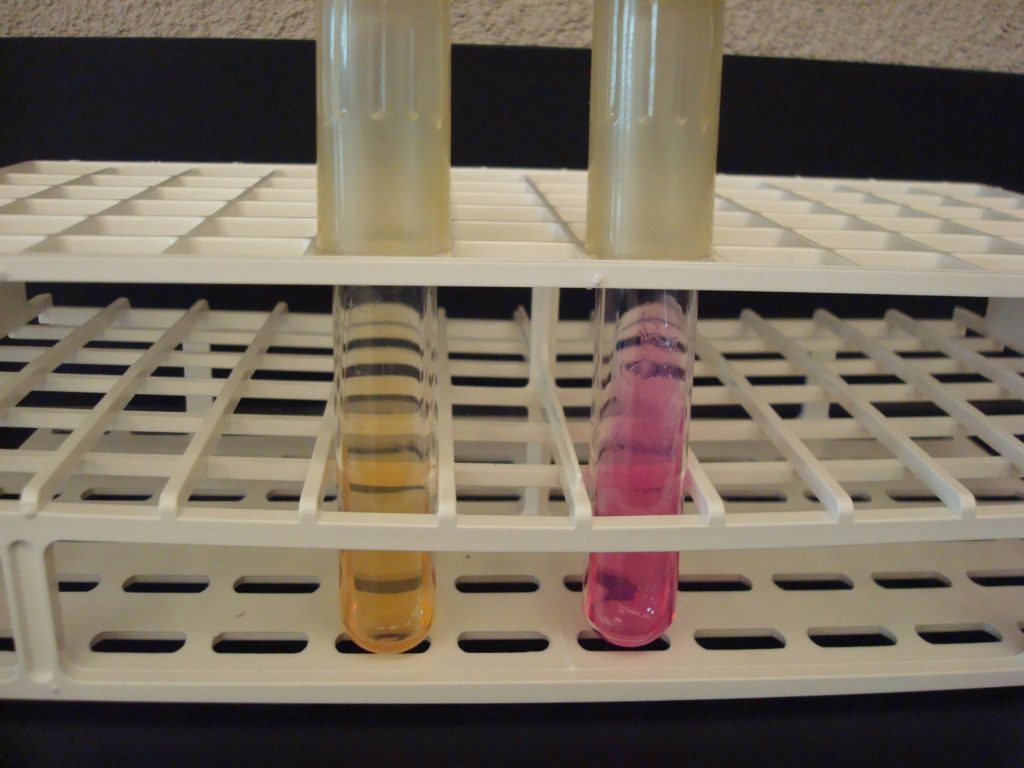
KLIGLER IRON AGAR (KIA)
KIA media contains two carbohydrates, glucose and lactose. It also has a phenol red pH indicator, ferrous sulfate, and peptones in the agar.
KIA media is used to determine if the organisms can ferment glucose with or without gas production. If the organism can ferment glucose, then KIA will also demonstrate if it can ferment lactose. The media can also determine if the organism can produce hydrogen sulfide.
If bacteria cannot ferment glucose, they are called “nonfermenters” and will not be able to ferment any other carbohydrates.
If bacteria can ferment glucose, they are called “fermenters” and can be differentiated by determining if it can also ferment lactose or produce hydrogen sulfide.
AFTER INCUBATION
Butt of KIA tube:
Yellow butt = Positive glucose fermentation (a black precipitate may mask the yellow color)
Red butt = Negative glucose fermentation
Gas production from glucose fermentation:
Bubbles or cracks in the medium may be visible, or the entire slant may be raised above the bottom of the test tube = Positive for gas (If bacteria cannot ferment glucose, they will not produce gas)
Slant of the KIA tube:
Yellow slant = Positive lactose fermentation
Red slant = Negative lactose fermentation
Hydrogen sulfide production:
Black precipitate in the media = Positive Hydrogen Sulfide Production
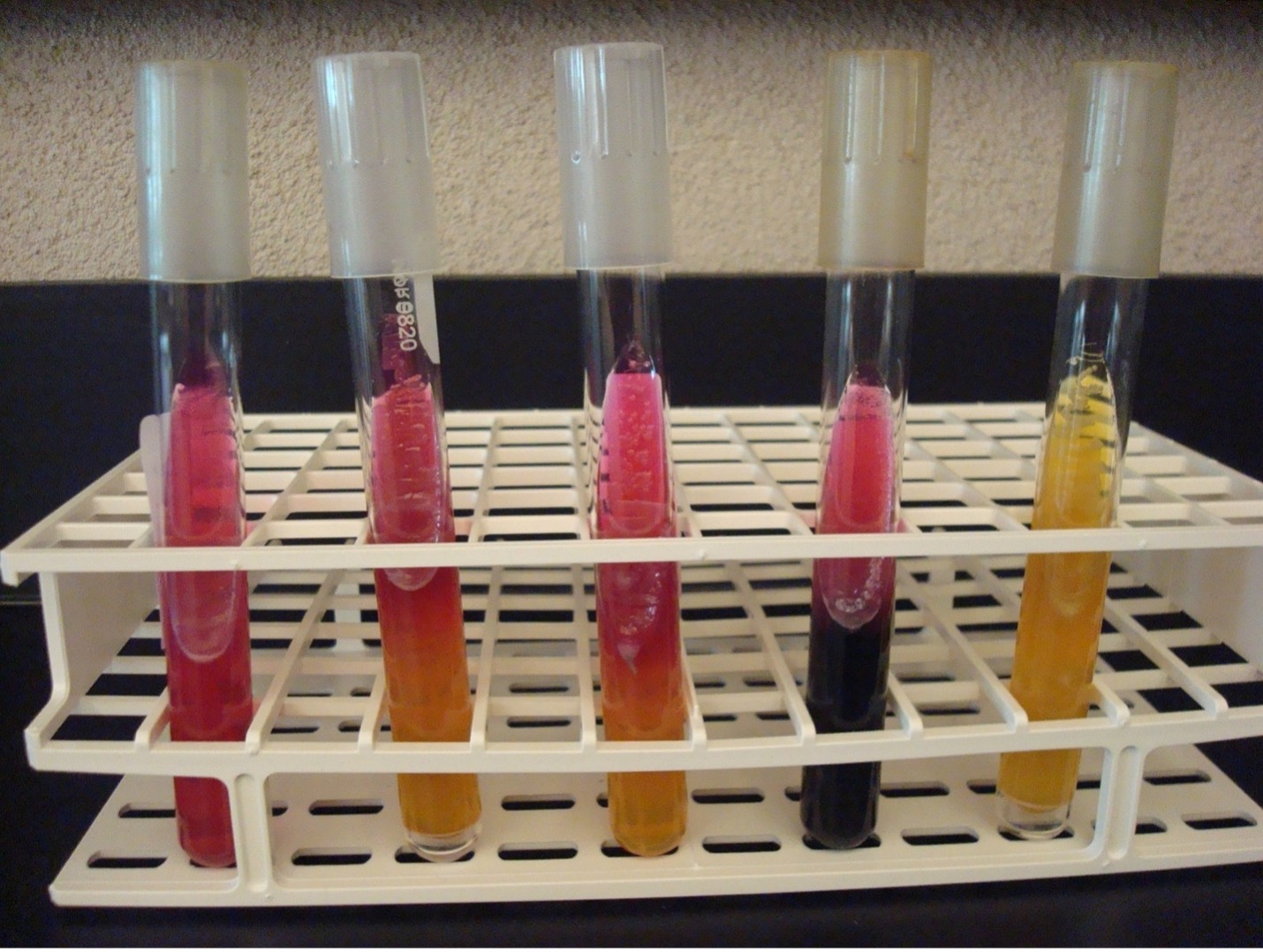
METHYL RED – VOGES PROSKAUER TEST
The MR-VP test is actually two separate tests that use one broth of glucose to determine the end product of glucose fermentation. Some bacteria ferment glucose using mixed acid fermentation pathway (detected in the MR test) while other bacteria ferment glucose via the butanediol fermentation pathway (detected in the VP test).
MRVP Broth (Glucose) —-> Pyruvic acid —-> Mixed acid fermentation pathway (MR +)
OR
MRVP Broth (Glucose) —-> Pyruvic acid —-> Butanediol fermentation pathway (VP+)
AFTER INCUBATION
METHYL RED TEST
10 drops of Methyl Red reagent is added to the methyl red tube. Methyl red is a pH indicator that will indicate the bacteria fermented glucose via the mixed acid fermentation pathway.
Red broth = MR Positive
Yellow broth = MR Negative

VOGES PROSKAUER TEST
15 drops of Barritt’s A reagent (alpha-napththol) and 5 drops of Barritt’s B (40% KOH) are added to the Voges Proskauer tube.
A positive reaction indicates the bacteria ferment glucose via the butanediol fermentation pathway.
Dark red-brown color at the top of the tube = VP Positive Yellow color at the top of the tube = VP Negative

NITRATE REDUCTION TEST
Can the bacteria produce ATP via anaerobic energy production and use nitrate ad the final member of the electron transport chain? If it can use nitrate as the final member of the electron transport chain, does it reduce the nitrate to nitrite or nitrogen gas? Nitrate broth is a differential media used to determine if an organism can reduce nitrate. Some bacteria can reduce nitrate (NO3) to nitrite (NO2) by producing the enzyme nitrate reductase. Other bacteria can reduce nitrate to nitrogen gas by also producing the enzyme nitrite reductase which reduces nitrite to nitrogen gas. Other organisms do not have the ability to reduce nitrate at all.
Nitrate ——> Nitrite ——> Nitrogen Gas
AFTER INCUBATION
10 drops of 0.8% sulfanilic acid and then add 10 drops of 0.6% N, N-Dimethyl-alpha-naphthylamine are added to the incubated nitrate broth. A dark red color indicates the organism used the nitrate reductase enzyme to reduce nitrate to nitrite. This is a positive test result, and no further testing is required.

If no red color occurs a wooden applicator stick is dipped in zinc powder and dropped it into the nitrate broth. The zinc powder reduces nitrate to nitrite. A red color after the addition of zinc is a negative test result. Nitrate was not reduced by the organism, it was reduced by zinc. If a red color does not occur after the addition of zinc, there was no nitrate for zinc to reduce. This is considered a positive result since the organism reduced nitrate to nitrogen gas.
No color = Positive (Nitrate was reduced to Nitrogen gas)
Red color = Negative (Nitrate was not reduced by the bacteria; zinc reduced the nitrate)

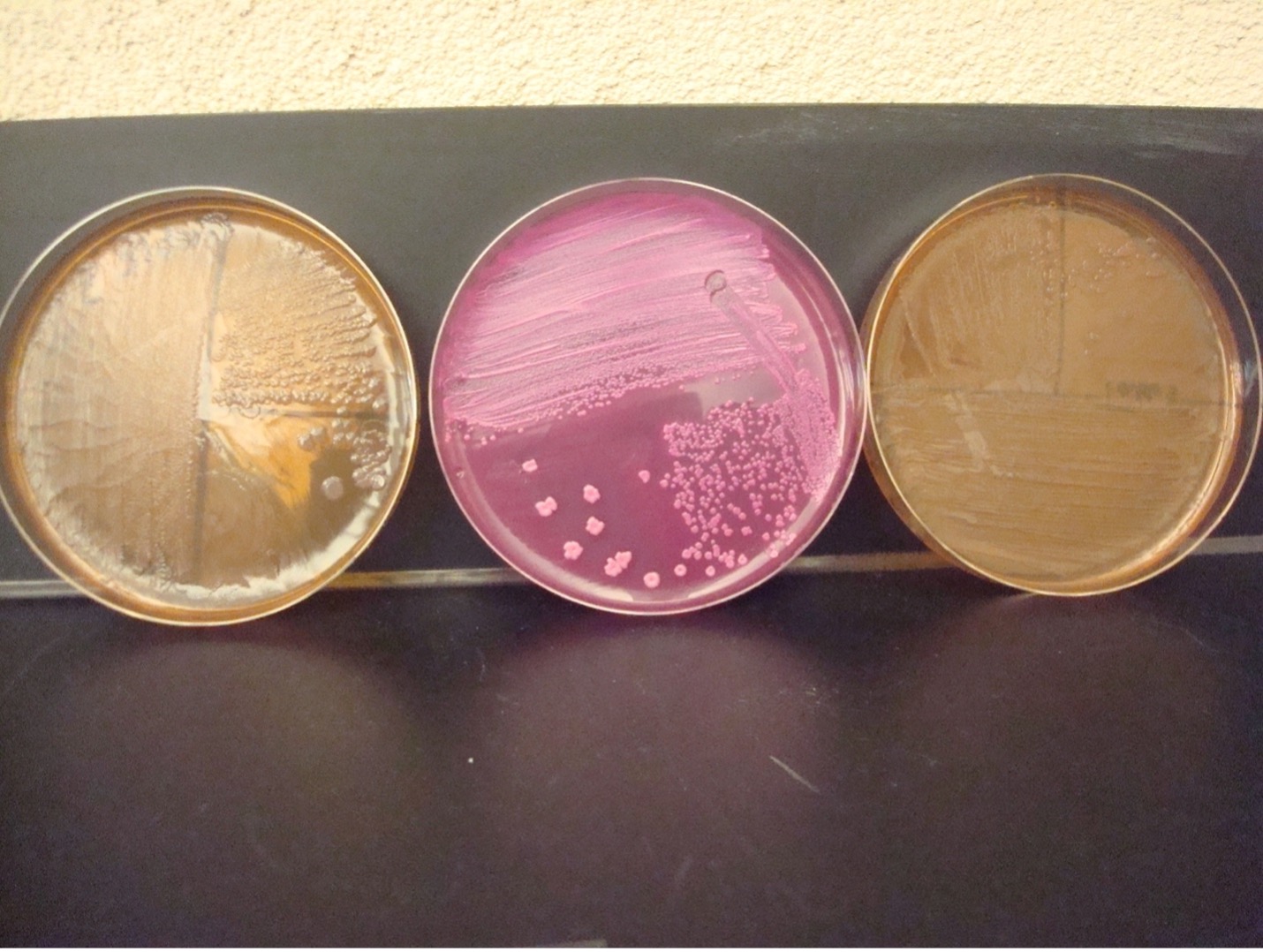
MACCONKEY AGAR (MAC)
MAC agar is a selective and differential media. It is used to grow Gram-negative bacilli and differentiate their ability to ferment lactose. MAC has bile salts and crystal violet dye which inhibit Gram-positive bacteria. The media also contains the carbohydrate lactose and a pH indicator. The Gram-negative bacilli that ferment lactose will produce acidic end products thereby changing the color of the pH indicator from clear to hot pink.
AFTER INCUBATION
Growth on media = Gram-negative bacilli
Determine the ability to ferment lactose:
Hot pink colonies = Positive for lactose fermentation
Clear colonies = Negative for lactose fermentation (some lactose negative colonies may have a slight pink color due to the transmittance of the media color through the colony)
identifications
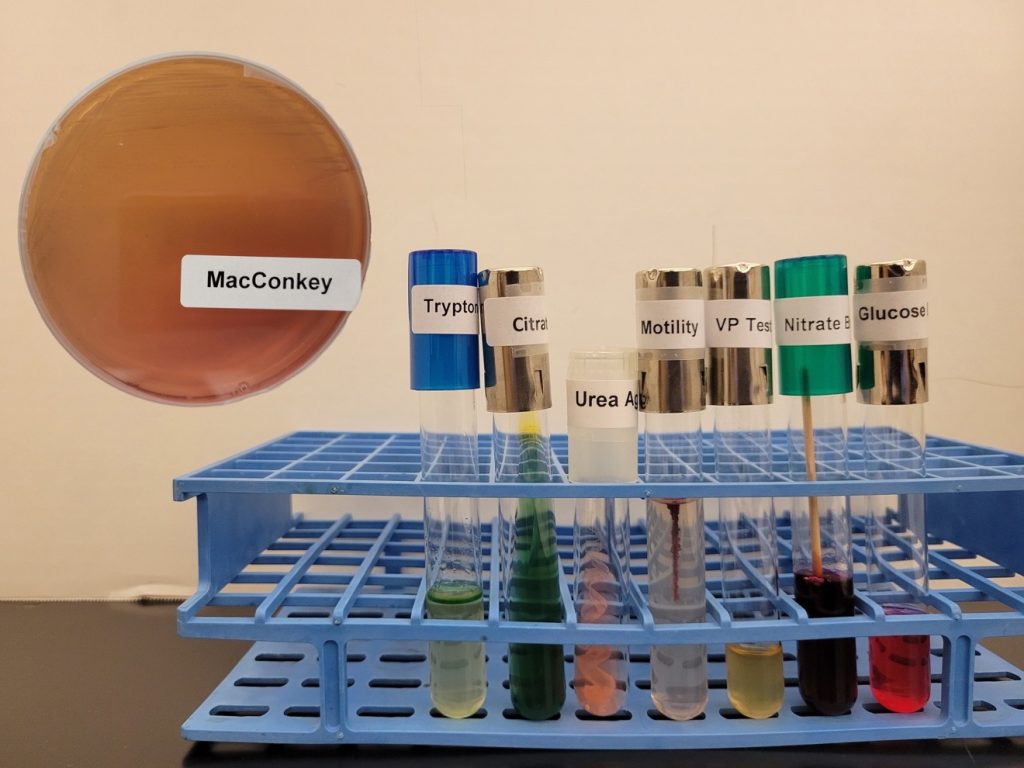
identification 1
Identify this organism using the Gram Negative dichotomous in the Dichotomous Key and Identifications Questions Document and the photo of media and biochemical test results below (if you need the nitrate test result, Alpha-naphylamine and sulfanilic acid AND Zinc have been added to the inoculated nitrate broth).

identification 2
Identify this organism using the Gram Negative dichotomous key in the Dichotomous Key and Identifications Questions Document and the photo of media and biochemical test results below (if you need the nitrate test result, Alpha-naphylamine and sulfanilic acid AND Zinc have been added to the inoculated nitrate broth).
DISCOVERIES IN MICROBIOLOGY
 DR. CÉSAR MILSTEIN
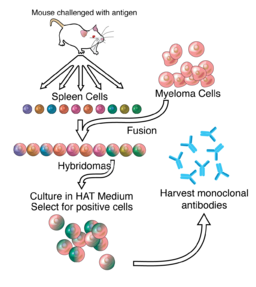
DR. CÉSAR MILSTEIN
In 1975, Argentine biochemist Dr. César Milstein was the first to produce monoclonal antibodies. Prior to the development of Dr. Milstein’s technique, scientists had struggled to produce large amounts of single antibody clones because of the difficulty of growing antibody producing B cells in culture. Dr. Milstein overcame this problem by fusing spleen cells derived from mice immunized with a specific antigen to immortalized myeloma cells, a technique that enabled production of antigen-specific antibody in culture indefinitely. His techniques were used to produce diagnostic tests, cancer treatments, disease treatment, vaccines, and blood and tissue typing.
