IDENTIFYING GRAM NEGATIVE BACTERIA DEMONSTRATION LAB
LEARNING OBJECTIVES
Distinguish between bacteria belonging in the Family Enterobacteriaceae from non-Enterobacteriaceae.
State the purpose and principle of the oxidase test, glucose carbohydrate fermentation tests and KIA.
Perform and interpret the oxidase test, nitrate reduction test, glucose carbohydrate fermentation tests and KIA.
State the significance of glucose fermentation and oxidase test in identifying Gram-negative bacilli.
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation.
Apply various laboratory techniques to identify types of microorganisms.
Identify structural characteristics of the major groups of microorganisms.
Compare and contrast prokaryotic cell and eukaryotic cell.
Compare and contrast the physiology and biochemistry of the various groups of microorganisms.
Gram negative bacilli comprise a vast array of bacteria, yet most clinically significant Gram-negative bacilli can be divided into two groups. One major group of Gram-negative bacilli is the Family Enterobacteriaceae which contain many genera of organisms such as E. coli, Klebsiella, Enterobacter and Proteus. These Gram-negative, facultative anaerobes are frequently called “enterics” since they are normally found in the intestinal tract of humans and other animals. The Gram-negative bacilli that are not in the Enterobacteriaceae family are composed of several families but are collectively called Non-Enterobacteriaceae or Nonfermenters (of glucose) to differentiate them from the Enterobacteriaceae. Several of the bacteria in this group include bacteria such as Pseudomonas aeruginosa, Alcaligenes faecalis and Acinetobacter baumanii. These bacteria are mostly found in the environment, yet some of these organisms can cause wound infections and serious life-threatening infections in immunocompromised patients.
The Enterobacteriaceae have four common characteristics:
- They are all Gram-negative bacilli
- They all ferment glucose
- They do not produce oxidase
- They all reduce nitrate to nitrite
The Non-Enterobacteriaceae also have three common characteristics:
- They are all Gram-negative
- They do not ferment glucose
- Many (but not all) are oxidase positive
Selective and Differential media and biochemical testS overview
NITRATE REDUCTION TEST
Can the bacteria produce ATP via anaerobic energy production and use nitrate ad the final member of the electron transport chain? If it can use nitrate as the final member of the electron transport chain, does it reduce the nitrate to nitrite or nitrogen gas? Nitrate broth is a differential media used to determine if an organism can reduce nitrate. Some bacteria can reduce nitrate (NO3) to nitrite (NO2) by producing the enzyme nitrate reductase. Other bacteria can reduce nitrate to nitrogen gas by also producing the enzyme nitrite reductase which reduces nitrite to nitrogen gas. Other organisms do not have the ability to reduce nitrate at all.
Nitrate ——> Nitrite ——> Nitrogen Gas
AFTER INCUBATION
10 drops of 0.8% sulfanilic acid and then add 10 drops of 0.6% N, N-Dimethyl-alpha-naphthylamine are added to the incubated nitrate broth. A dark red color indicates the organism used the nitrate reductase enzyme to reduce nitrate to nitrite. This is a positive test result, and no further testing is required.

If no red color occurs a wooden applicator stick is dipped in zinc powder and dropped it into the nitrate broth. The zinc powder reduces nitrate to nitrite. A red color after the addition of zinc is a negative test result. Nitrate was not reduced by the organism, it was reduced by zinc. If a red color does not occur after the addition of zinc, there was no nitrate for zinc to reduce. This is considered a positive result since the organism reduced nitrate to nitrogen gas.
No color = Positive (Nitrate was reduced to Nitrogen gas)
Red color = Negative (Nitrate was not reduced by the bacteria)

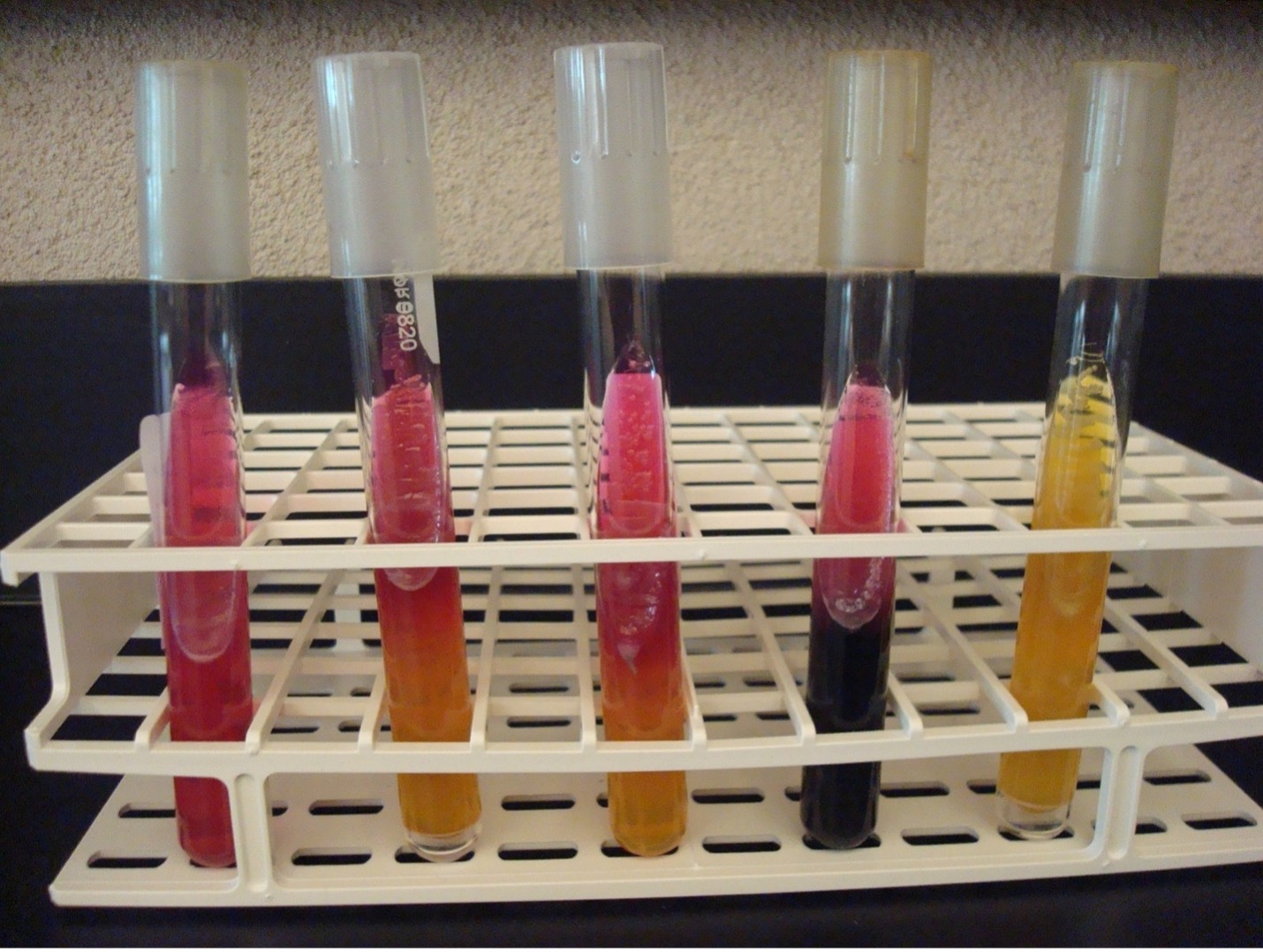
KLIGLER IRON AGAR (KIA)
KIA media contains two carbohydrates, glucose and lactose. It also has a phenol red pH indicator, ferrous sulfate, and peptones in the agar.
KIA media is used to determine if the organisms can ferment glucose with or without gas production. If the organism can ferment glucose, then KIA will also demonstrate if it can ferment lactose. The media can also determine if the organism can produce hydrogen sulfide.
If bacteria cannot ferment glucose, they are called “nonfermenters” and will not be able to ferment any other carbohydrates.
If bacteria can ferment glucose, they are called “fermenters” and can be differentiated by determining if it can also ferment lactose or produce hydrogen sulfide.
AFTER INCUBATION
Butt of KIA tube:
Yellow butt = Positive glucose fermentation (a black precipitate may mask the yellow color)
Red butt = Negative glucose fermentation
Gas production from glucose fermentation:
Bubbles or cracks in the medium may be visible, or the entire slant may be raised above the bottom of the test tube = Positive for gas (If bacteria cannot ferment glucose, they will not produce gas)
Slant of the KIA tube:
Yellow slant = Positive lactose fermentation
Red slant = Negative lactose fermentation
Hydrogen sulfide production:
Black precipitate in the media = Positive Hydrogen Sulfide Production
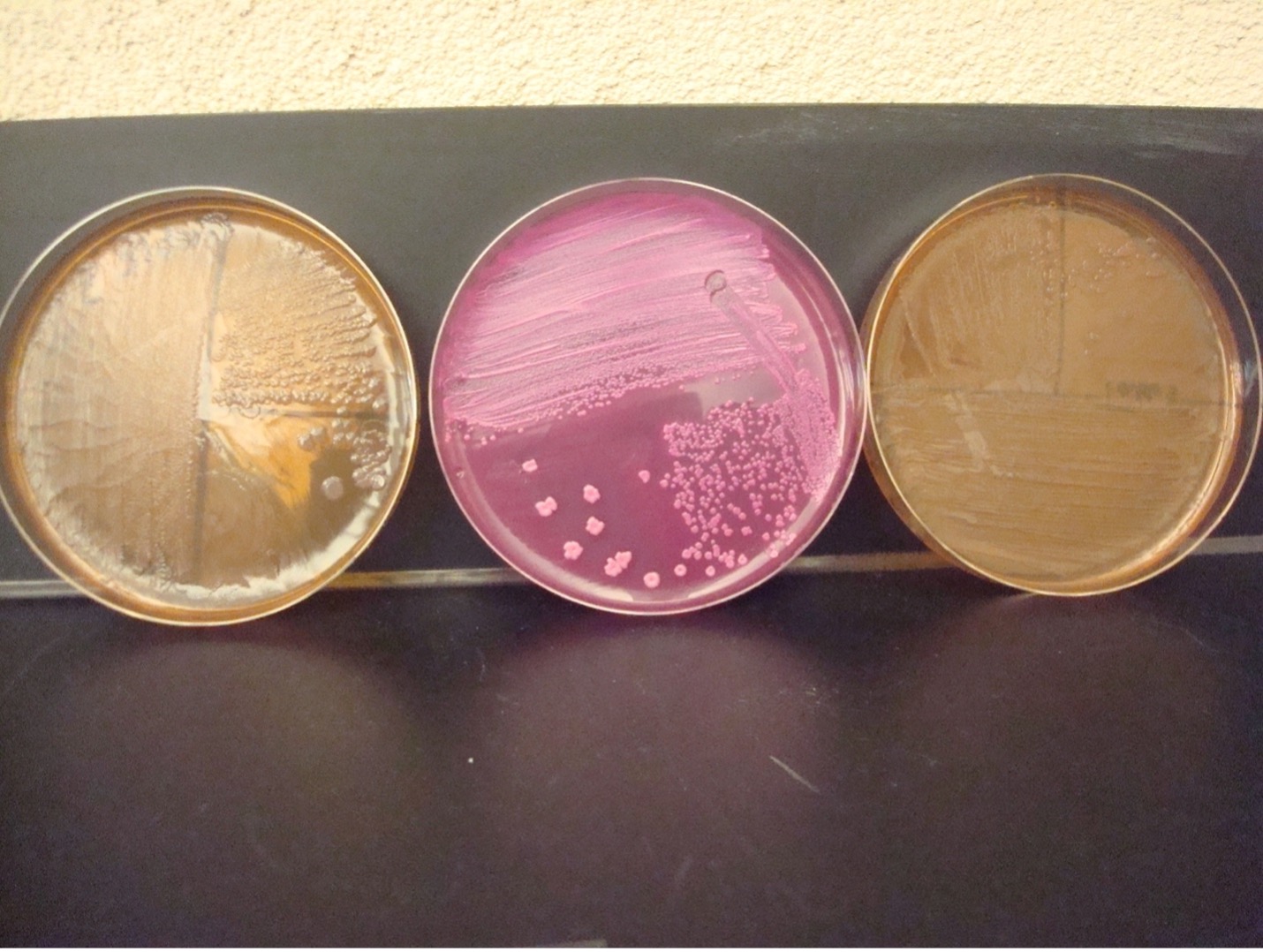
MACCONKEY AGAR (MAC)
MAC agar is a selective and differential media. It is used to grow Gram-negative bacilli and differentiate their ability to ferment lactose. MAC has bile salts and crystal violet dye which inhibit Gram-positive bacteria. The media also contains the carbohydrate lactose and a pH indicator. The Gram-negative bacilli that ferment lactose will produce acidic end products thereby changing the color of the pH indicator from clear to hot pink.
AFTER INCUBATION
Growth on media = Gram-negative bacilli
Determine the ability to ferment lactose:
Hot pink colonies = Positive for lactose fermentation
Clear colonies = Negative for lactose fermentation (some lactose negative colonies may have a slight pink color due to the transmittance of the media color through the colony)
OXIDASE TEST
This test determines if the bacteria can produce the enzyme cytochrome oxidase which transfers electrons in the electron transport chain in cellular respiration. The organism to be tested must be grown on non-inhibitory media such as KIA or TSA because the production of oxidase is easily inhibited by selective media. A purple color on the strip or swab within 20 seconds is a positive result.
Purple color change = Positive
No color change within 20 seconds = Negative

identifying gram negative unknowns 1, 2, 3, and 4
Interpret the media and biochemical test results below for Gram Negative Unknowns 1, 2, 3, AND 4. Complete the data table and the identifications in the Identifying Gram Positive Unknowns Question Document.
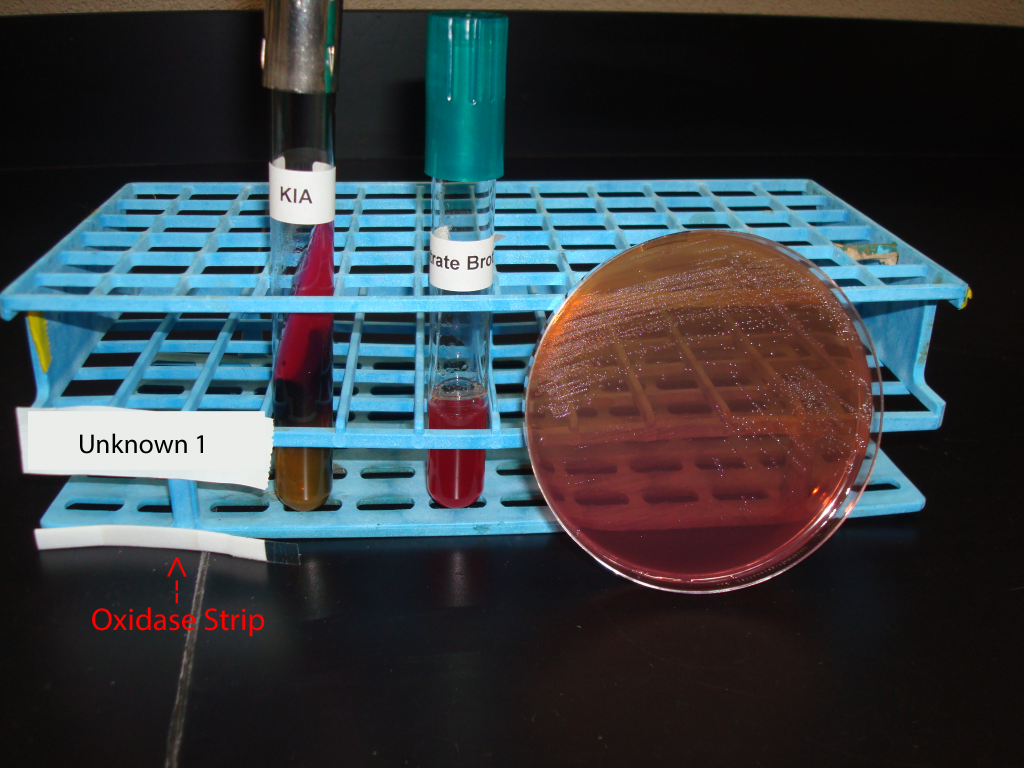
UNKNOWN 1 MEDIA AND TEST RESULTS
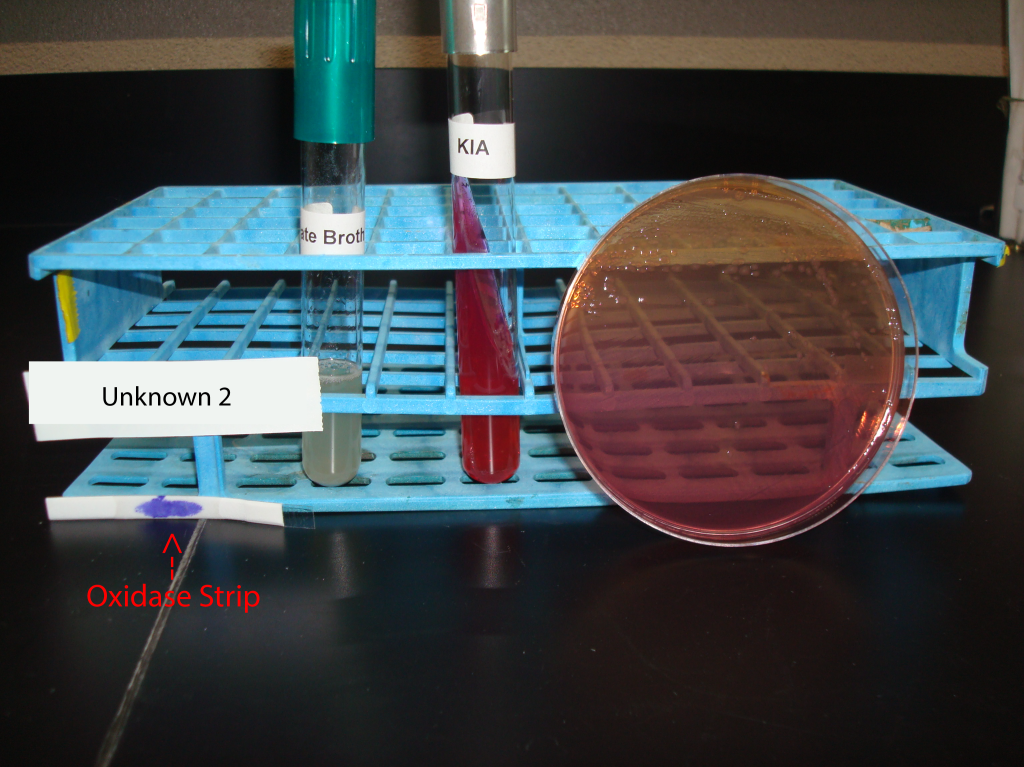
UNKNOWN 2 MEDIA AND TEST RESULTS
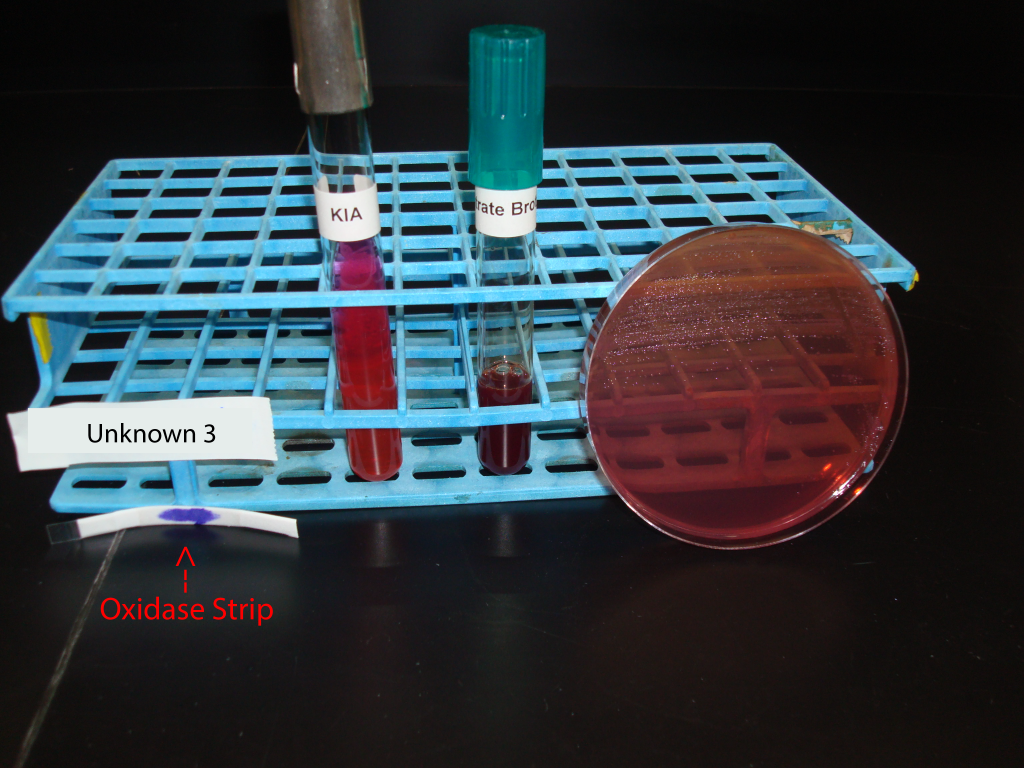
UNKNOWN 3 MEDIA AND TEST RESULTS
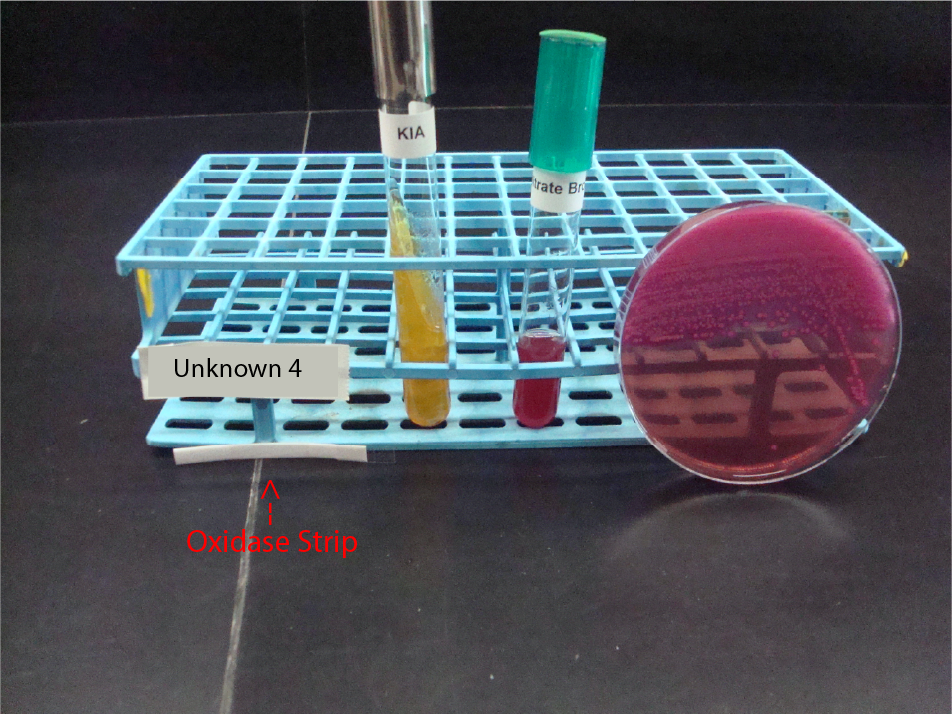
UNKNOWN 4 MEDIA AND TEST RESULTS
DISCOVERIES IN MICROBIOLOGY
DR. EMIL von BEHRING
In 1890, German physiologist Dr. Emil von Behring published an article with Dr. Kitasato Shibasaburō reporting that they had developed “ antitoxins” against both diphtheria and tetanus. They had injected diphtheria and tetanus toxins into guinea-pigs, goats and horses. The animals developed antitoxins (now known to contain antibodies) in their serum. These antitoxins could protect against and cure the diseases in non-immunized animals. In 1892 Dr. Behring began the first human trials of the diphtheria antitoxin, but they were unsuccessful. After the production and quantitation of the antitoxin was improved, in 1894 humans were successfully treated with the antitoxin.
antitoxins” against both diphtheria and tetanus. They had injected diphtheria and tetanus toxins into guinea-pigs, goats and horses. The animals developed antitoxins (now known to contain antibodies) in their serum. These antitoxins could protect against and cure the diseases in non-immunized animals. In 1892 Dr. Behring began the first human trials of the diphtheria antitoxin, but they were unsuccessful. After the production and quantitation of the antitoxin was improved, in 1894 humans were successfully treated with the antitoxin.
