3.1 Elements and Chemical Bonding
Elements

There are 118 elements that make up all matter as we know it. To review, each one of those elements is made up of atoms. An atom is composed of protons and neutrons in its central nucleus and electrons in its outer shells. The number of protons that a certain atom has depends on the atomic number of the element it composes. For instance, there are 6 protons in the nucleus of a carbon atom because its atomic number is six. There are 82 protons in the nucleus of a lead atom because its atomic number is 82.
Bonding
Electrons are more interesting because they can migrate to and from different atoms to form chemical bonds. A chemical bond is an ongoing attraction between two atoms that forms a molecule, and it can happen in a number of ways. To understand bonds, first you must understand how electrons move.
In the above video, you have learned about Ionic and Covalent bonding. As the video also indicated, there are other types of bonding that can happen between atoms. Let’s briefly review them below!
Metallic Bonding – The highly positively charged nucleus of metallic elements as well as their surrounding negatively charged electron shells causes two or more atoms of metals to bond together.

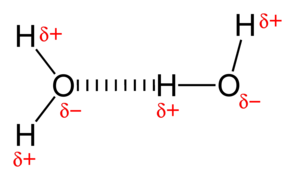
Hydrogen Bonding – A weak electrostatic bond, often between water molecules; i.e., where a partially positive hydrogen atom in one water molecule is attracted to a partially negative oxygen atom in another water molecule. In minerals, this happens between sheets of clays in the atomic structure.
Chemical Groups in Minerals
Because elements make up all existing matter, they also make up all existing minerals. Minerals are special because they have a defined chemical formula – that means we can always predict what a certain mineral is made out of. For example, if you found a quartz crystal in your backyard, you would know that it is made out of the elements arranged in the chemical formula, SiO2. If you traveled to Tibet and found another quartz crystal at the base of the Himalayas, this crystal would also be made out of SiO2! A given mineral, whether it is quartz, calcite, gold, or pyrite, has a predictable chemical formula that does not change!
Minerals are typically categorized based on their chemical formula, or the main types of elements we find in them. Look at the table below to see how minerals are differently labeled.
| Mineral Group Name | Chemical Composition | How It’s Distinguished | Examples |
| Native Elements | Single Elements | One element makes up its chemical formula | Sulfur, Silver, Gold |
| Silicates | SiO2 Family | Has some version of “SiO” in its chemical formula | Quartz, Feldspars, Micas |
| Carbonates | CO3 Family | Has “CO3” in its chemical formula | Calcite |
| Sulfates | SO4 Family | Has “SO4” in its chemical formula | Gypsum |
| Oxides | Oxygen Anion | Has “O2“, “O3” or “O” in its formula *with none of the preceding groups, such as SiO2 or CO3 | Hematite, Magnetite |
| Sulfides | Sulfur Anion | Has “S” at the end of its formula | Pyrite, Galena |
| Halides | Chloride or Fluoride Anion | Has “Cl”, “F” “I”, or “Br” in its formula, usually at the end | Halite, Fluorite |
IMPORTANT: Make sure you scroll through all three questions to the end of the slides to see your results in order to get points for this activity!

