3.5 Mineral Habit and Unique Identifying Properties
Mineral Habit
Habit is the shape and texture of the mineral. It can refer to the expression of a crystal shape or the shape of multiple crystals aggregated or bunched together. Besides color, it is often the first thing you might notice about a mineral. Often when asked to draw a crystal, we will picture a traditional prism shape below:

This type of crystal habit is called prismatic. Prismatic crystals are elongated but have very clearly-defined crystal faces. However, there are many different types of crystal habits we can use to identify other types of minerals! Check them out in the table below! [1]
Now that you are familiar with some of the basic crystal forms, let’s test your identification skills!
Sample 1

Sample 2

Sample 3

Sample 4

Sample 5

Other Unique Identifying Properties
Some minerals, whether it is by chemical composition or the arrangement of their atomic structure, have unique properties that make them much more easily identifiable. Some glow brightly under UV light, others are magnetic. Some fizz when dilute acid is dripped onto their surfaces, and some are extremely dangerous! There’s a wide range of unusual and interesting things that minerals can do!
Magnetism

Some minerals are magnetic/attract iron-containing materials! Perhaps the most well-known of these magnetic minerals is commonly called lodestone, or magnetite(Fe3O4). Testing a mineral for magnetism is pretty straightforward; you simply use the mineral to attract a paperclip or a small magnet.
Some more weakly-magnetic minerals include:
- Babingtonite (Ca2Fe2Si5O14OH)
- Chromite (FeCr2O4)
- Ferberite (FeWO4)
- Pyrrhotite (FeS)
Fluorescence
When some minerals are exposed to higher frequency UV light, the atoms within them are excited to a higher energy state. As those atoms relax back down to a lower energy state, they’ll emit light at a new wavelength, which can mean that we’ll see beautiful and bright colors! Here are some examples:

Just because one sample of a mineral is fluorescent does not mean another one will be! For example, you can have a sample of calcite from Franklin, NJ that fluoresces under a UV light, but a calcite sample from your backyard might not! It is the tiny trace elements within a mineral’s structure that often cause this reaction!
Optical Properties
We learned that some minerals can be completely transparent, but even if we can view images through certain crystals, they can appear different or distorted! Here are two good examples of optical properties in minerals.
Calcite is well-known to “double” images if you look through clear, transparent samples of this crystal. This property is called “Double Refraction”.
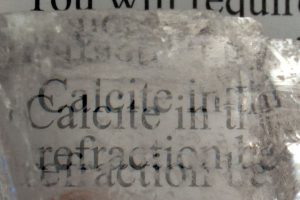
Translucent ulexite can transmit an entire image when polished. This unique ability to transmit images has given ulexite the nickname “TV Stone”.

Effervescence
Effervescence is a mineral’s reaction to acid. Geologists can often identify certain minerals by dripping small amounts of dilute hydrochloric acid on them. This is called the “acid test”.
The reason that Calcite (CaCO3) reacts so strongly to acid is that the carbonate (CO3) in the mineral dissolves and becomes a gas, CO2! This is an important thing to keep in mind for later modules as CO2 is an important greenhouse gas, that in natural quantities, the Earth can regulate by storing carbonate-containing rocks!
Danger!
Some minerals can be extremely hazardous to human health. Unfortunately, many of these can be bright and visually attractive minerals. It’s always important in the field to protect yourself by researching the area–oftentimes, other geologists will report if there is a mineral quarry known for producing radioactive minerals or toxic minerals. You should also always cover your airways to protect yourself if a mineral is loose, fibrous, or powdery.

In the activities below, there are visual examples of dangerous minerals mixed among safe minerals. These are (when applicable) accompanied by chemical formulas. Examine the images and the chemical formulas to determine which likely contains something that is unsafe for humans!
Sample 1: Sulfur, S

Sample 2: Realgar, As4S4

Sample 3: Uranophane, Ca(UO2)2[HSiO4]2·5H2O

Sample 4: Vanadinite, Pb5(VO4)3Cl

HINT: Think about which ELEMENTS in the Periodic Table are radioactive and which ELEMENTS are poisonous!








