3.3 A Visual Guide to Common Minerals
Charlene Estrada
Now that we know some of the identifying properties of minerals, we will go over some of the most common minerals we encounter on the Earth. Many of these are the main ingredients of rocks, and others have been extraordinarily useful in our society. What makes these minerals unique?
Video 3.3.1. The following brief lecture will show you the most common diagnostic properties of minerals (11:29).
Native Elements
The native elements are a group of minerals that are composed of only one element. Many of these play a vital role in the global economy and industry. Table 3.3.1 contains common examples of native element minerals.
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
|
Gold (Au) Precious Metal 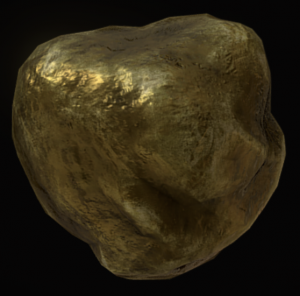 |
Brassy yellow | Deep yellow | Metallic | Uneven Fracture | 2.5 – 3.0 |
|
Silver (Ag) Precious Metal
Figure 3.3.2. “bonsai branch_ silver, copper, crystal gem” by subarcticmike is licensed under CC BY 2.0 |
Metallic gray | Light Gray | Metallic | None | 2.5 -3.0 |
|
Copper (Cu) Economic Mineral, Industrial Use in Technology and Ores
Figure 3.3.3. “Large native copper amygdule (Mesoproterozoic, 1.05-1.06 Ga; Ahmeek Mine, Ahmeek, Upper Peninsula of Michigan, USA) 1” by James St. John is licensed under CC BY 2.0 |
Bronze red-brown | Red-Brown | Metallic | Uneven Fracture | 2.5 – 3.0 |
|
Diamond (C) Precious Mineral, Conflict Resource  |
Clear, blue, brown, gray | None | Adamantine | Fracture | 10 |
|
Graphite (C) Economic Mineral, pencil “lead”
Figure 3.3.5. “Graphite” by James St. John is licensed under CC BY 2.0 |
Light to dark gray | Dark gray | Greasy | Fracture | 1.0 – 2.0 |
|
Sulfur (S) Economic Mineral used in explosives  |
Bright yellow | Colorless | Dull to Vitreous | Uneven to Conchoidal Fracture | 1.5 – 2.5 |
Silicates
Minerals of the silicate group are composed of some combination of silicon (Si) and oxygen (O). Silicate minerals make up the majority of the planet, both on the surface and within the interior. There are many types of silicate minerals, but below are the most common varieties.
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
| Quartz (SiO2)
Use in glassmaking, significant ingredient in sand  |
Variable | White | Vitreous | Conchoidal Fracture | 7.0 |
| Potassium Feldspar (KAlSi3O8)
Figure 3.3.8. “Orthoclase” by Photographer: John Bosworth is licensed under CC BY 4.0 |
Orange-pink (orthoclase) or blue-green (microcline) | White | Vitreous to Dull | 2 cleavage directions at 90° | 6.0 |
| Plagioclase (NaAlSi3O8 to CaAl2Si2O8)
Figure 3.3.9. “Moonstone (iridescent peristerite-oligoclase feldspar) (Chupa Pegmatite Field, Mesoproterozoic, 1.75 to 2.10 Ga; at or near Chupa Bay, Karelia, Russia) 2” by James St. John is licensed under CC BY 2.0 |
Commonly white, sometimes blue | White | Vitreous | 2 cleavage directions, non-90° | 6.0 – 6.5 |
| Muscovite (Mica) KAl2(AlSi3O10)(F,OH)2
Flaky, pulls apart in sheets  |
Clear, white, gray, tan | White | Vitreous | Basal Cleavage | 2.0 – 2.5 (but breaks very easily!) |
| Biotite (Mica) K(Mg,Fe)3(AlSi3O10)(F,OH)2
Flaky, pulls apart in sheets  |
Black, dark gray, black-brown | White | Vitreous | Basal Cleavage | 2.5 – 3.0 (but breaks very easily!) |
| Kaolinite (Clay) Al2Si2O5(OH)4
Key ingredient in porcelain/china
Figure 3.3.12. “Kaolinite (Cretaceous; Twiggs County, Georgia, USA)” by James St. John is licensed under CC BY 2.0 |
Commonly white or tan | White | Dull/Earthy | Basal Cleavage | 2.0 – 2.5 |
| Talc (Clay) Mg3Si4O10(OH)2
Use in cosmetics and paint
Figure 3.3.13. “Talc schist 2” by James St. John is licensed under CC BY 2.0 |
White, gray, clear, light green, brown | White | Waxy to greasy | Basal Cleavage | 1.0 |
Almandine (Garnet) Fe2+3Al2Si3O12
 |
Dark red to purplish-red | White | Vitreous | Conchoidal Fracture | 7.0 -7.5 |
| Hornblende (Amphibole) (Ca,Na)23(Mg,Fe,Al)5(Al,Si)8O22(OH,F)2
Figure 3.3.15. “File:Magnesio-hornblende (cropped).png” by Creator:Robert Lavinsky is licensed under CC BY-SA 3.0 |
Black to dark green | Light gray to white | Vitreous to Dull | 2 Cleavage directions at 56° and 124°
Uneven Fracture |
5.0 – 6.0 |
| Enstatite (Pyroxene) MgSiO3
Figure 3.3.16. Pyroxene, var. Enstatite
|
Gray, green, brown, yellow | Gray | Vitreous | 2 Cleavage directions at 90°
Uneven Fracture |
5.0 – 6.0 |
| Olivine (Mg,Fe)SiO4
AKA Peridot  |
Green, sometimes Yellow-green | None | Vitreous | Conchoidal Fracture | 6.5 – 7.0 |
Carbonates
Minerals in the carbonate group all have the elements carbon (C) and oxygen (O) arranged into what is called the carbonate anion, which is a carbon bonded with three oxygens: CO3–. Carbonate minerals play a key role in storing the world’s carbon dioxide, a greenhouse gas, in solid form. They also make up the “hard parts” of some animals such as shells in marine life. Table 3.3.3 shows some of the carbonate minerals you will most commonly encounter:
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
| Calcite CaCO3
Main ingredient in limestone, fizzes with dilute acid, double refraction, sometimes fluoresces  |
Variable, but commonly colorless or white | White | Vitreous | 3 Cleavage Directions; non-90° | 3.0 |
| Dolomite CaMg(CO3)2
Material in fossilized shells
Figure 3.3.19. “File:Dolomite Eugui MNHN Minéralogie.jpg” by Marie-Lan Taÿ Pamart is licensed under CC BY-SA 4.0 |
White, colorless, gray, brown | White | Vitreous | 3 Cleavage Directions; non-90°; Rhombohedral | 3.5 – 4.0 |
| Malachite Cu2CO3(OH)2
Always green  |
Green | Green | Vitreous to Dull | Basal Cleavage
Uneven Fracture |
3.5 – 4.0 |
| Azurite Cu3(CO3)2(OH)2
Always blue
Figure 3.3.21. “File:Azurite from China.jpg” by E. Hunt is licensed under CC BY-SA 2.5 |
Blue | Light Blue | Vitreous to Dull | 2 Directions | 3.5 – 4.0 |
Sulfates
Sulfate minerals are made of the elements sulfur (S) and oxygen (O) that are arranged into a sulfate ion: SO4–. Some of the most common sulfate minerals form within hot, dry environments when bodies of water, such as lakes, evaporate. These minerals build our homes and cities. Here are some of their properties below:
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
| Gypsum CaSO4·2H2O
Use in construction materials  |
Colorless, white, tan or yellowish | White | Vitreous, silky or waxy | 3 Cleavage Directions, but one direction is perfect
Conchoidal Fracture |
2.0 |
| Anhydrite CaSO4
Stable, dehydrated version of gypsum
Figure 3.3.23. “Anhydrite.” by Holly Leighanne. is licensed under CC BY 2.0 |
Variable: white, gray, pale blue, colorless | White | Vitreous to greasy | 3 Cleavage Directions, almost cubic | 3.5 |
Oxides
Oxides are a mineral group that are defined by a combination of a metal cation bonded with oxygen (O). A lot of these minerals tend to be metallic and have found use in industry as sources of metal ores. However, this group also includes hydroxide minerals, which are minerals that contain oxygen bonded with hydrogen. Some of these minerals are used in our everyday lives!
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
| Magnetite Fe2+Fe3+2O4
Magnetic mineral
Figure 3.3.24. “File:Magnetite-118736.jpg” by Rob Lavinsky, iRocks.com licensed under CC BY-SA 3.0 |
Black, Dark Gray | Black | Metallic | Uneven Fracture | 5.5 – 6.5 |
| Hematite Fe2O3
Heme means “blood”.  |
Dark Gray to Red-Brown | Red-Brown to Bright Red | Metallic | Uneven Fracture | 5.5 – 6.5 |
| Corundum
Al2O3 AKA “Ruby” (when red), “Sapphire” (when blue)  |
Reddish pink to blue for gemstones, also brown to gray | Colorless | Adamantine to Vitreous | Fracture only | 9.0 |
| Ice H2O
Only a mineral at freezing temperatures.
Figure 3.3.27. “Ice-cubes.” by rawdonfox is licensed under CC BY 2.0 |
Colorless, White, Pale Blue | White | Vitreous | Conchoidal Fracture | 1.5 |
Sulfides
Minerals within the sulfide group all contain a metallic cation that is bonded with sulfur (S) as an anion. Nearly all the world’s ore materials can be found within the sulfide group. Metallic sulfides form in association with volcanic activity.
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
| Pyrite FeS2
AKA “Fool’s Gold”  |
Brassy Yellow | Dark Gray to Brownish-Black | Metallic | Uneven Fracture | 6.0 – 6.5 |
| Galena PbS
Heavy/ High density.
Figure 3.3.29. “Galena (Missouri, USA)” by James St. John is licensed under CC BY 2.0 |
Silver Gray to Dark Gray | Dark Gray | Metallic | 3 Cleavage Directions at 90°; Cubic
Near-Conchoidal Fracture |
2.5 – 3.0 |
Halides
Minerals in the halide group are composed of a cation element bonded with a halogen anion element. Some of these minerals are known as salts because, like several sulfates, they form when water evaporates in hot, arid environments. One of the most well-known halides is something that we use in our kitchen every day!
| MINERAL | COLOR(S) | STREAK | LUSTER | BREAKAGE | HARDNESS |
| Halite NaCl
Rock Salt, salty taste  |
Colorless, white | White | Vitreous | 3 directions of perfect cleavage at 90°; Cubic | 2.0 – 2.5 |
| Fluorite CaF2
Often Fluorescent
Figure 3.3.31. “Fluorite (Denton Mine, near Cave-in-Rock, Illinois, USA) 2” by James St. John is licensed under CC BY 2.0 |
Variable | White | Vitreous | 6 directions; Octahedral | 4.0 |
a solid, inorganic, and crystalline substance that has a predictable chemical composition and form by natural processes.
A group of minerals composed only of a single chemical element.
a metallic, brassy-yellow mineral made only of the element gold (Au). It is a precious metal in the world economy.
A luster that has the appearance of a polished metal surface reflecting light.
the breakage of a mineral (or sometimes, a rock) that is not along a cleavage plane.
a grayish-white metallic mineral composed only of the element silver (Ag). It is a precious metal used by the global economy.
A metallic, brownish-red mineral composed only of the element copper (Cu). It is used widely in the global economy in industry and technology.
An extremely hard mineral composed only of carbon (C). It is a precious mineral that can take on a glassy to brilliant luster and it is sometimes a conflict resource.
Brilliant, very reflective version of glassy luster. Often applies to diamonds and other highly polished gemstones.
A very soft, grayish mineral composed only of carbon (C). It is used in the global economy as pencil "lead".
A nonmetallic luster that appears oily.
A bright, yellow-colored mineral that often smells of rotten eggs. It is composed only of the element sulfur (S), and it often mined for its use in explosive materials.
Reflective and glassy luster.
The breakage of a rock or mineral that forms smooth, curved surfaces.
A mineral class that is primarily composed or defined by silicon (Si) and oxygen (O).
The color of a mineral when crushed or scratched into a powder.
The way in which light reflects from a mineral's surface. The observation of luster can help identify a mineral.
a measure of a mineral's durability or resistance to being scratched.
A very common rock-forming silicate mineral with formula SiO2.
A group of silicate minerals which represents the most abundant mineral class of the continental crust.
A type of potassium feldspar that is orange-pink in color. It is more abundant of the K-Feldspars and often found in granite.
A type of potassium-feldspar that is blue-green and more rare within the K-Feldspar class.
A class of light-colored feldspar that forms at higher temperatures and can be either sodium (Na) or calcium (Ca) rich.
A common mineral in the mica group that is light grayish-brown. It is flaky and peels apart in sheets.
A group of silicate minerals called the "sheet" silicates. Mica minerals have their atomic structure arranged to easily pull apart as sheets in one direction.
A type of mica that is black or dark-colored and is typically found in igneous and metamorphic rocks.
Al2Si2O5(OH)4
A type of clay silicate mineral that is white or very light-colored. It has historically been used for making porcelain.
A group of silicate minerals that typically forms after existing rocks and minerals, such as feldspars, are chemically altered by water.
A magnesium (Mg)-rich clay mineral that is very soft and light-colored. This mineral is often used in cosmetics and paint.
Fe2+3Al2Si3O12
A dark red garnet mineral composed of iron and aluminum.
X3Y2(SiO4)3
A group of hard silicate minerals that form under high pressures and temperatures. Garnets can have dodecahedral habit and are commonly found in metamorphic rocks, such as schist.
an exclusively dark-colored group of amphibole minerals that often composes igneous rocks and metamorphic rocks.
a class of silicate minerals that are usually dark and form long crystals (as prisms or needles). Amphiboles are often magnesium (Mg) and iron (Fe) rich in composition.
MgSiO3
A common type of pyroxene mineral that has a magnesium (Mg) rich composition.
(Mg,Ca,Fe)SiO3
A class of dark-colored silicate minerals that form under high temperatures and/or pressures. These form in igneous and metamorphic rocks.
(Mg,Fe)SiO4
A class of silicate mineral that forms at high temperatures and can have a magnesium (Mg) or iron (Fe) rich composition.
a gemstone commonly made from the forsterite (Mg-dominated) variety of the mineral olivine
A mineral group composed of the carbonate (CO3--) anion.
CaCO3
A carbonate mineral that strongly reacts with dilute acid. Calcite composes the sedimentary rock limestone and composes the skeletons of some ocean life.
A carbonate mineral, CaMg(CO3)2, that is more resistant to weathering by acid at room temperatures. Dolomite often composes the material of fossilized shells.
Cu2CO3(OH)2
A green, copper-carbonate mineral that is sometimes used for decorative purposes.
A type of cleavage in which the mineral splits along a single plane or direction.
Cu3(CO3)2(OH)2
A blue, copper carbonate mineral that is sometimes used for decorative purposes.
A mineral group composed of the sulfate (SO4--) anion.
A common evaporite mineral that precipitates in dry or arid environments near evaporating water. It has the formula CaSO4 * 2H2O.
A nonmetallic luster with a cloth-like sheen or is finely-fibrous.
A nonmetallic luster with a candle-like appearance that weakly reflect light on its surface.
CaSO4
A sulfate mineral composed mainly of calcium. This mineral is a more thermally stable, dehydrated version of gypsum.
A class of minerals with a chemical composition of a metallic element bound with oxygen.
Fe2+Fe3+2O4
A dark gray, oxide mineral that is iron-rich. Magnetite is strongly magnetic and attracts iron-rich materials.
Fe2O3
A gray to rust-brown oxide mineral that is iron (Fe)-rich. The reddish color forms from the oxidation, or rusting, of iron by the atmosphere.
A hard (Mohs 9), oxide mineral that, when cut and polished can either become a ruby (red or pink) or a sapphire (blue) depending on its color.
A mineral compound characterized by the linkage of sulfur with a metal or semi-metal such as galena, PbS, or pyrite, FeS2.
FeS2
A brassy yellow metallic mineral from the sulfide class. Pyrite is also known as "Fool's Gold" for its golden color.
PbS
A gray, metallic mineral of the sulfide class. Galena is very dense and feels heavy when held.
A mineral group composed of the chloride (Cl-) or fluoride (F-) anion.
elements found in the 17th column of the Periodic Table of Elements. Examples include chloride, fluoride, bromine, and iodine.
NaCl
A salty, halide mineral that is often colorless or white. Halite is commonly known as rock salt and used globally as a condiment. It tends to form as cubic-shaped grains or crystals.
CaF2
A halide mineral that often fluoresces under ultraviolet light. Fluorite can be almost any color, and it will sometimes form octahedrally-shaped crystals.

















