10.2 Coal
Jeff Simpson and Carolina Londono Michel
Learning Objectives – By the end of this chapter, you should be able to do the following.
- Explain the conditions under which coal formed, when it formed, and what it formed from.
- Explain why anthracite coal is preferred as a fuel source to peat or lignite.
- Explain the changes that occur when changing from peat to lignite to bituminous to anthracite.
- Describe the different types of coal mines.
- Describe the primary ways that we use coal.
- Explain how electricity is generated from coal.
- Explain the use of and problems with the disposal of coal ash as well as one possible new use.
- Explain how our civilization has been built by burning coal.
- List several health and environmental, health, and social consequences associated wit coal extraction.
The black lump below, a piece of coal, has made our modern civilization possible. Societies around the world have burned coal for centuries as it has been a plentiful, widely distributed, fuel with high energy content. However, the extraction and burning of coal also has produced severe consequences for the environment and societies.

What Is Coal and How Did It Form?
Video 10.2.1 – How Coal Made Us Rich and Why it Needs to Go (8:21)
Main Points from the Video
Coal powered the industrial revolution leading to our modern civilization.
Coal still is a primary source of energy in many countries.
Burning coal contributes to climate change and millions of deaths around the world each year.
China and India are the largest consumers of coal while other countries are shutting down most of their coal plants.
Many new coal plants will shut down before the end of their normal life, becoming stranded assets – a financial liability.
The coal industry is working to delay a transition away from coal.
Coal resources in Wales often are cited as one reason for the rise of Britain (and later the United States) in the industrial revolution.1 Coal, the first fossil fuel to be widely used, forms mostly on land in swampy areas next to rivers and deltas in areas with humid tropical to temperate climates. The vigorous growth of vegetation leads to an abundance of organic matter that accumulates within stagnant water and thus does not decay and oxidize. This condition in which dead organic matter is submerged in oxygen-poor water must be maintained for centuries to millennia in order for enough material to accumulate to form a thick layer. At some point, the swamp deposit is covered with more sediment – typically because a river changes its course or sea level rises. As more sediments are added, the organic matter becomes compressed and heated changing to a form we call peat. Peat has been used as a fuel for centuries.


Over time, given pressure and heat, peat can turn into the low-grade type of coal called lignite. Lignite forms at depths between a few 100 m and 1,500 m and temperatures up to about 50°C. Between 1,000 m to 5,000 m depth and temperatures up to 150°C m, bituminous coal forms (Figure 20.3.1d). At depths beyond 5,000 m and temperatures over 150°C, anthracite coal forms. As the coal becomes more compressed and heated, water, volatiles, and oxygen are driven out and the percentage of carbon increases, leading to a more desirable fuel product. Much of the coal being burned today in India is low-grade coal and results in significant air pollution, health issues, and deaths.


How is Coal Mined – Strip Mines
When a seam of coal coal is closer than ~100′ to the surface, the overburden, the rock covering the coal, is blasted away and the coal is scooped up. This type of mine requires the fewest employees and can extract vast amounts of coal in a short time. However, this works only when the sedimentary rock layers that contain the coal are close to the surface such as in the Black Thunder Coal Mine in Wyoming, USA.

Video 10.2.2 – World’s Biggest Mine: Inside US Coal at the North Antelope Rochelle Mine, Wyoming (5:17)
Main Points from the Video
Most of the coal that mining companies hope to extract must stay in the ground if we want limit damage from climate change.
How is Coal Mined – Mountaintop Removal
When coal-bearing sedimentary rocks are in an area that has been incised or cut by streams rather than being flat as shown above, a process called mountaintop removal is used as in this area near Danville, West Virginia, USA. The Land of Mountaintop Removal (2:57) by Smithsonian shows how 500 mountains and 3200 streams in the Appalachian Mountains near West Virginia have been destroyed by mountaintop removal. But this process, like strip mining, is more cost effective and safer than underground mining. (The Shocking Danger of Mountaintop Removal | Michael Hendryx TED Talk (13:44) is a fascinating look at the findings of science and the work of mining companies to cover up those findings.)
Video 10.2.3 -How Mountaintop Mining Affects Life and Landscape in West Virginia (9:24)
Main Points from the Video
Mountaintop removal leads to environmental destruction, polluted water, destroyed ecosystem, and short-term profits.
How is Coal Mined – Underground Mining
When coal is too far below the surface to access easily, vertical shafts are drilled until the coal seam is reached. Then horizontal drifts or tunnels allow miners to remove the coal from the mine. This form of mining is the most dangerous due the possibility of a mine collapse or the ignition of methane leaking from the coal seam. Additionally, miners are exposed to airborne silica dust (from the rock) and coal. Over years, this leads to black lung disease and silicosis. (See Mining the Navajo Nation and Big Money, Black Lung, and Doctors for the Coal Companies.)
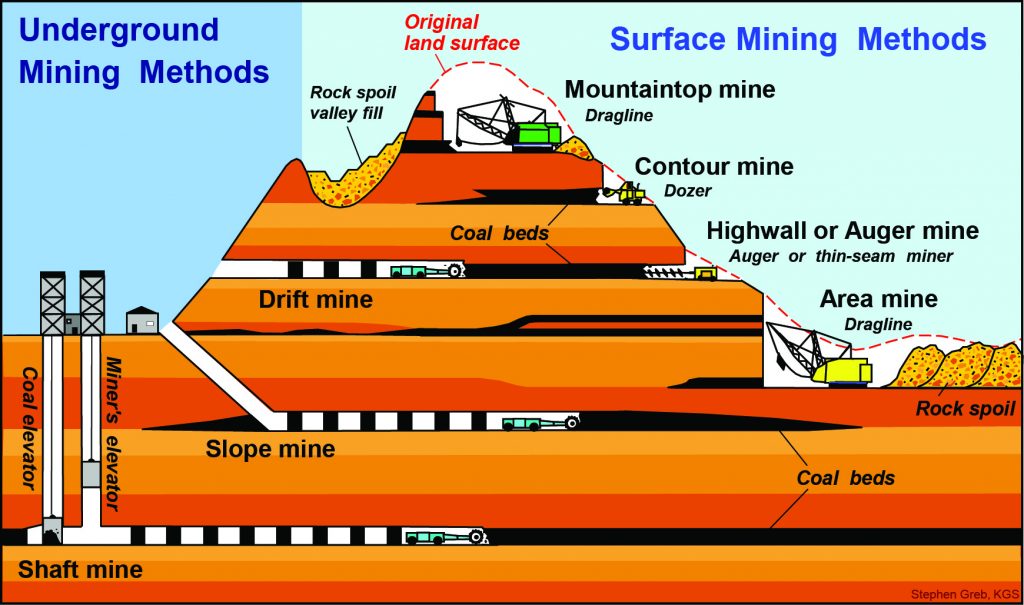

Where Coal Is Found?
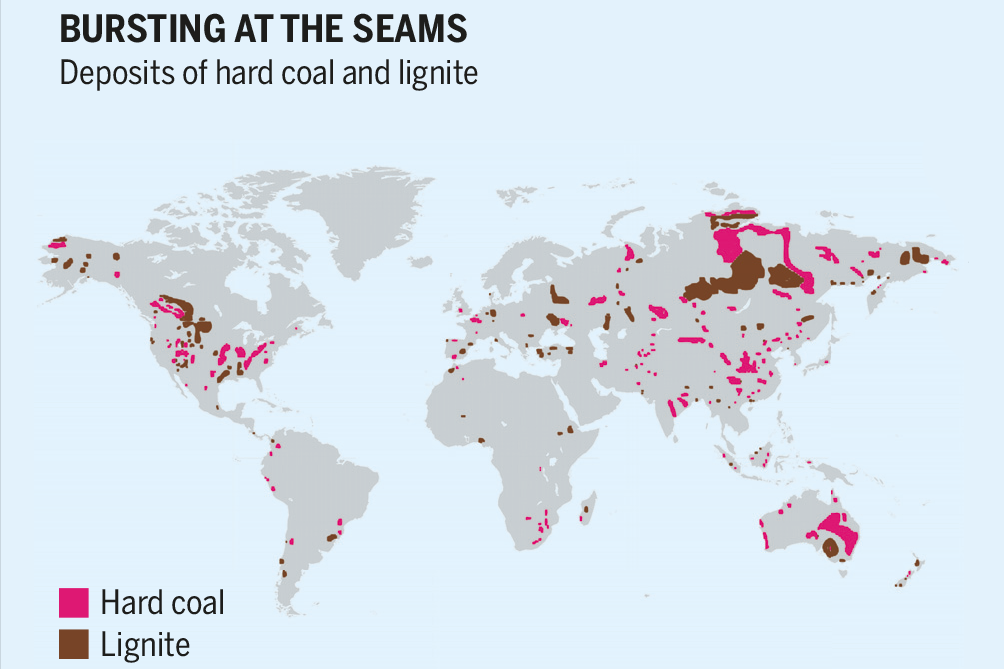
Coal is mined today near the surface where, during the Carboniferous period, the conditions were correct for plants to grow, die, and stack up. Not all countries have access to coal. This unequal distribution of a prized fossil fuel led Germany during World War II to look far and wide for more coal to fuel its war effort.
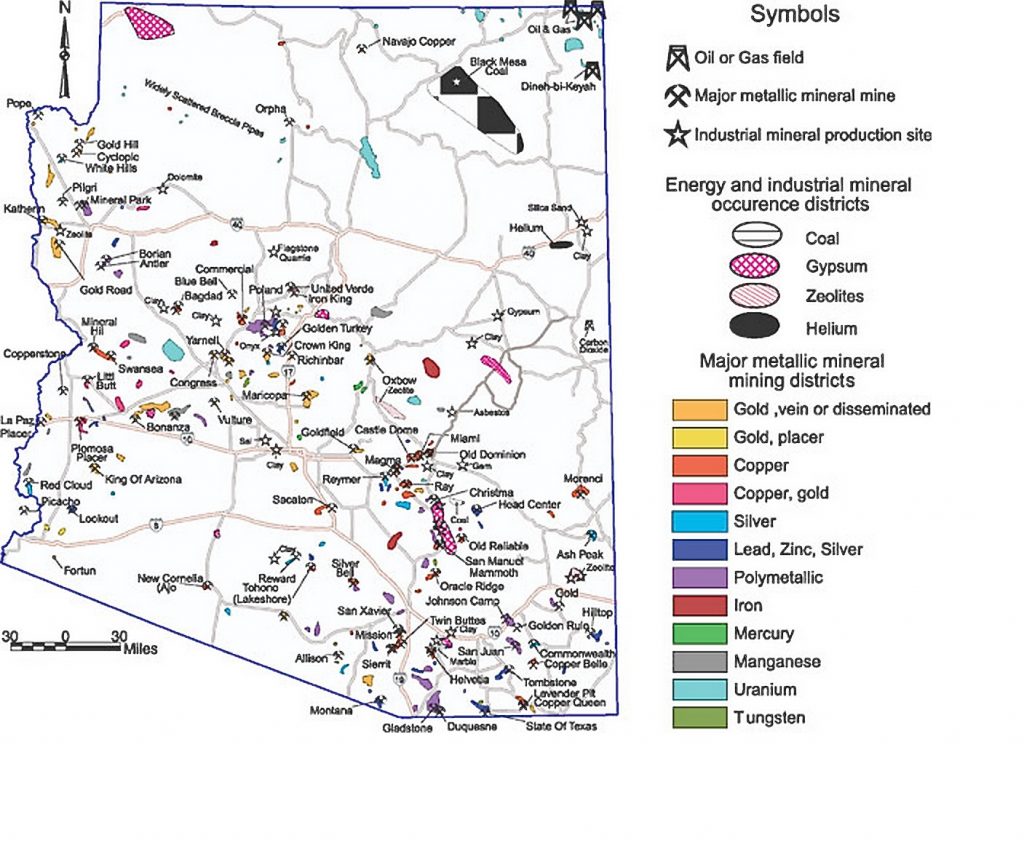
The coal deposits and mines in northeastern Arizona fueled the Navajo Generating Station near Lake Powell and Page, AZ. The mining of those same deposits required the extensive pumping of groundwater, causing many Navajo families to have to haul water and leading to lung disease for many of the workers. But mining coal also provided some jobs and a near 50-year boost to the local economy.
Coal Power Plants
Once coal is mined, it is used primarily in smelting, the making of steel, or – the major use at 2/3 – producing electricity. As of 2022, worldwide there were about 6593 coal-fired power stations generating ~ 1/3 of the world’s electricity. Many of these plants are not fully utilized, some existing only for statistical purposes. Though the amount of coal being burned is increasing slowly, that trend is about to reverse.
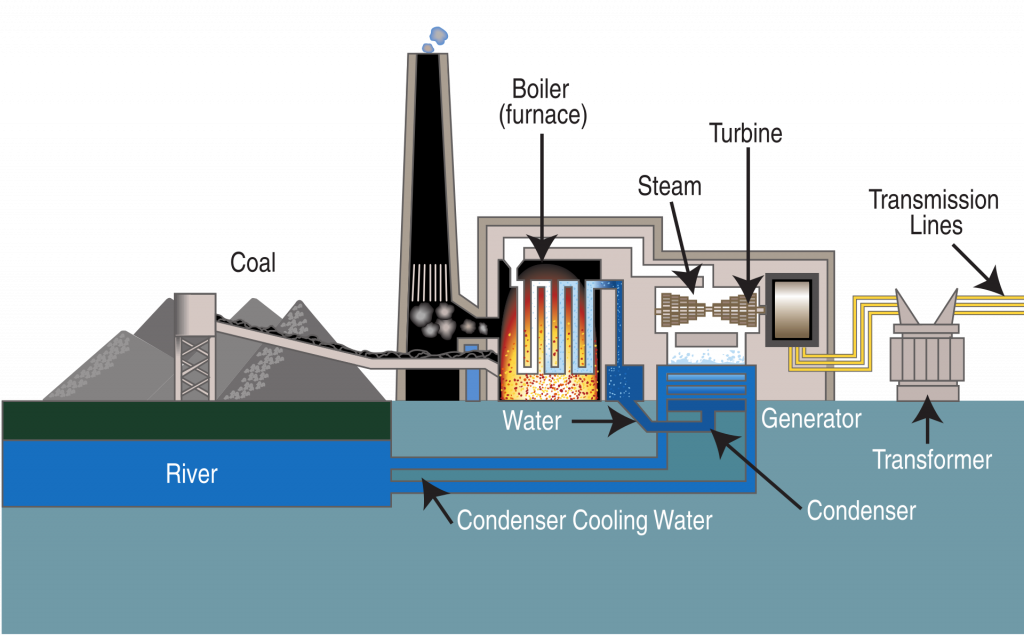
To generate electricity in a coal power plant, the coal is pulverized to a fine powder and injected into a boiler. A 500 MW plant may shoot 250 tonnes per hour under full load. That is 152 pounds of coal per second. Each of the three former coal units at the Navajo Generating Station near Page, Arizona when fully operating used 300 pounds/second.
As the coal contains some silica from the rock where it was mined, the very expensive pipes in a coal plant are worn down quickly and have to be replaced.
The boilers create steam which turns a turbine which turns a generator to make electricity in a process that may have begun 400,000,000 years ago when the sun’s energy combined elements and molecules in growing plants, after which the plants died, were buried in the absence of oxygen, and were pressed and heated. Nearly 400,000,000 years later, we break apart those original solar bonds to release the chemical energy which becomes thermal energy which becomes mechanical energy and, finally electrical energy. In the process a lot of pollution is released.
Coal-fired power stations emit over 10 Gt of CO2 each year (~1/5 of world greenhouse gas emissions or 40% of the energy emissions) and are the single largest cause of climate change. More than half of all the coal-fired electricity in the world is generated in China, though China is beginning to consolidate and phase out coal plants. Coal plants remain profitable only because the externalized environmental and health costs are not included. If damage to our health and environment were included in the cost of coal power, coal would be phased out quickly. We allow our atmosphere to be a free dumping ground. Our leaders ignore this issue. As more countries move away from coal, hundreds of billions of dollars that have been invested in coal plants will become stranded assets, something that concerns investors.
Coal Ash
Coal ash is what is left over from the burning of coal in coal-fired power plants. Coal ash is one of the largest sources of industrial waste in the US with ~130 million tons of coal ash generated in 2014. Some coal ash is used in making wallboard, concrete, roofing materials, and bricks in a way that minimizes the toxic materials contained from escaping into the surrounding environment. Other coal ash is used as structural fills/embankments where the stability of the toxic materials is more in question. About 60% of all coal is placed in on-site impoundments or landfills where is will be contained “forever”… hopefully. However, major coal ash spills in Kingston Tennessee and Eden, NC have shown this is not the case. The Kingston spill [GE] alone cost over $1,000,000,000 to clean up. In the coming decades, more coal ash sites will suffer the same fate. Is burning coal to generate electricity really worthwhile? Would you want a coal-fired power plant near your house?

Coal ash includes a) fly ash, b) bottom ash, c) boiler slag and flue gas desulfurization material. Fly ash is fine, powdery material composed mostly of silica made from the burning of finely ground coal in a boiler. Without containment, that ash would fly up the smoke stack. Bottom ash is coarse, angular ash too large to be carried up into the smoke stacks so that it collects in the bottom of the coal furnace. Boiler slag is molten bottom ash that turns into pellets that have a smooth glassy appearance after being cooled with water. Flue gas desulfurization material contains remains from the process of reducing sulfur dioxide emissions from a coal-fired boiler. It can be a wet sludge consisting of calcium sulfite or calcium sulfate or a dry powered material that is a mixture of sulfites and sulfates and other products.
Coal ash contains contaminants like mercury, cadmium, and arsenic which can pollute streams, groundwater, and our atmosphere. The waste left behind from burning coal will be with us for thousands of millennia. Contamination of groundwater from coal ash has occurred in Texas. A report found that the groundwater around coal-fired plants across the state contains levels of pollutants like arsenic, boron, cobalt or lithium that would make it unsafe for human consumption. It also found that almost none of the impoundments where plants dispose of spent coal are lined properly — one of the requirements of the 2015 Coal Ash Rule. Despite rules being in place, many fossil fuel companies try to work around them or just ignore them and often are not held accountable.
Average Heavy (Toxic) Metal Content of Coal
| Mercury (Hg) | 0.10±0.01 ppm |
| Arsenic (As) | 1.4 – 71 ppm |
| Selenium (Se) | 3 ppm |
However, this is some potential good news related to coal ash. We need a reliable supply of some materials called rare earth elements for our new gadgets such as smartphones, LED lights, electric motors, and batteries. Despite their name, rare earth elements (REEs) not that rare, but they are concentrated in only a few locations leading to supply chain issues and international tensions. A possible solution this limitation is to mine coal ash. Researchers have reported a simple method for recovering REEs from coal fly ash which has similar elemental concentrations as the raw ores. Until now, extracting these precious elements was complicated and hazardous. Researchers report that environmentally benign and reusable ionic liquids can dissolve rare-earth oxides over other metal oxides. The ionic liquid can be recovered easily. Researchers have extracted >77% of the REEs from fresh coal ash and 97% from weathered ash that had spent years in a storage pond.
Coal can also be considered as an economic source of strategically important elements, such as Ge, Ga, U, V, and Se, rare earth elements such as Y, Sc, Nb, Au, Ag, and Re, and base metals Al and Mg.
Coal and Methane Releases
The process of mining coal releases large amounts of methane into the atmosphere. Even abandoned coal mines emit methane into the atmosphere. This is a concern as methane as a greenhouse gas is far more powerful than carbon dioxide.
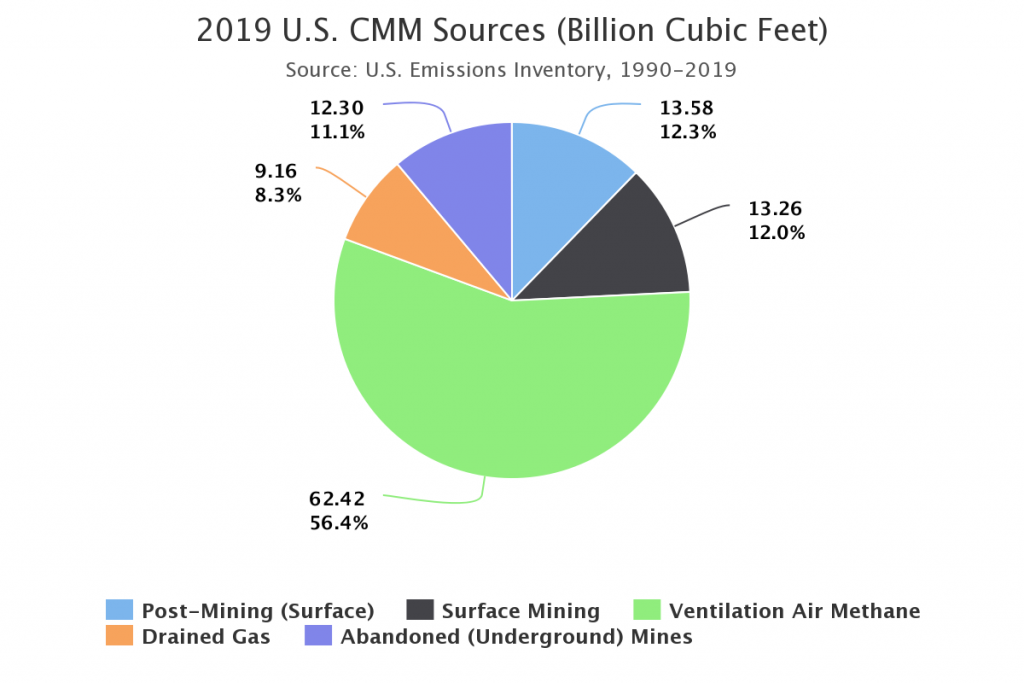
What is the Future for Coal?
As late as the 1980s, coal provided over half the electricity in the US, but by 2022 that fraction had fallen to 22%. The burning of coal for electricity in the US continues to decline, though there will be some instability due to the variable price of natural gas.
In the industrialized world, coal use is declining, though China and India still use vast quantities of coal. Many in the industrialized world point at China and ask, “Why should we stop using coal when China isn’t?” The simple answer is that we have exported much of our manufacturing and thus our coal pollution to other countries. We get affordable items, people have jobs, but our pollution is exported and not identified with us.
As the world moves toward cleaner sources of energy, though there will continue to be a need for coal in the making of steel, both for heat as a source of carbon to combine with iron to make steel. Eventually, hydrogen may be able to take the place of coal in industrial heat.
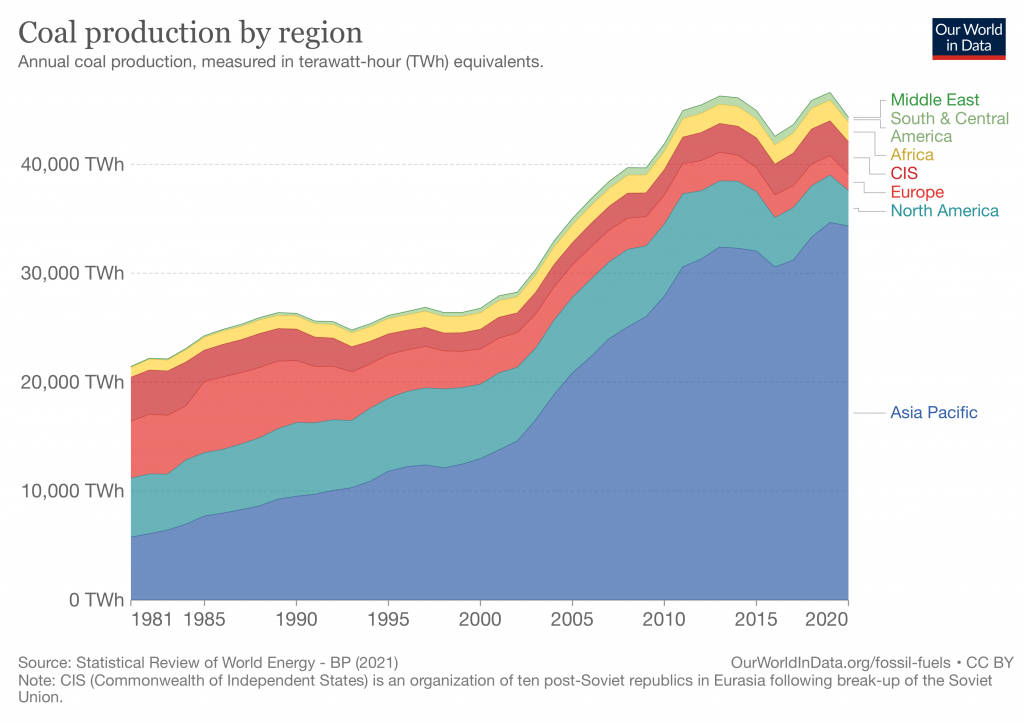
The amount of coal consumed each second by the world is equivalent to ~400 barrels of oil, but the future of coal is less bright.
Video 10.2.4 – Why Coal Has No Future (2:21) – Note: Electricity generated by renewable sources is increasing each year.
Main Points from the Video
Coal is the most expensive energy source.
A coal plant ramps up very slowly compared to a natural gas power plant.
The amount of electricity provided by wind and solar is increasing. The amount provided by coal is decreasing.
Coal and Health
The burning of this much coal results in many illnesses and early deaths, mainly from air pollution. Peer-reviewed research published in Nature showed that emissions from fossil fuels killed over 1,000,000 people around the world in 2017 with half of those deaths attributable to emissions from coal.
Coal miners often suffer from silicosis and black lung disease, and, in some cases, the coal mining companies deny worker compensation.
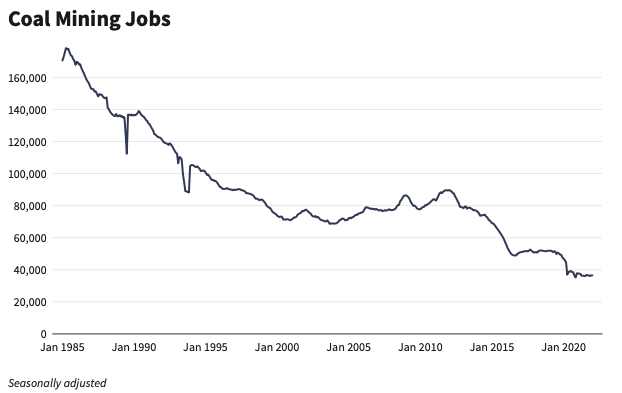
Coal and Jobs
The mining of coal once employed almost 180,000 workers in the US. As of 2023, that number was less than 38,000 and falling rapidly as power plants that once used coal are turning to methane, wind, solar, and nuclear and adding storage.
Advantages of Coal
1. It creates some jobs.
2. It is a concentrated source of energy.
3. It can run an electric power plant 24 hours a day.
Disadvantages of Coal
1. The burning of coal is a major source of CO2.
2. The burning of coal releases toxic metals (As, Hg, Cd) into the air, water and soil.
3. The burning of coal releases small particulates, PM 2.5, that get into our water, blood and brain.
4. The mining of coal is destructive to habitats including mountaintops and streams.
5. Coal generates over 100,000,000 tons of ash/year containing Hg, U, Th, As and other heavy metals in the US.
6. Coal electric power plants are slow to ramp up and down.
7. Coal plants are not very efficient. Only ~40% of the energy in a piece of coal becomes electricity. The coal plant itself is one of the biggest users of its own energy, using up to 8% of its own energy by some accounts. (Running all those motors and pumps requires a lot of electricity.)
8. Coal plants are most often placed far from cities, and the electrical power must travel long distances through transmission lines, incurring more losses and resulting in even lower efficiency.
9. Operating a coal plant requires more maintenance and more manpower than other power plants thus decreasing profitability.
10. Sulfur from burning coal leads to acid rain, though scrubbers in the US can remove much of the sulfur.
11. The health of people living near coal mines and plants is affected adversely.
12. Coal-fired power plants cause nearly 24,000 premature deaths/yr in the US. Annual health costs in Europe from coal plants are €42.8 billion, or $55 billion. These externalized costs are not paid by the utility but are borne by the citizens.
13. Subsidence leading to infrastructure damage can occur above underground coal mines.
14. Coal-fired power plants release more radioactivity via their emissions than a nuclear power plant in normal operation.
Low-grade type of coal with less than 70% carbon
a medium-grade type of coal with 70 to 92% carbon
a high grade of coal (92 to 98% carbon) that is formed from deep burial and weak metamorphism

