14.1 Unique Properties of Water
Katherine J. Megivern
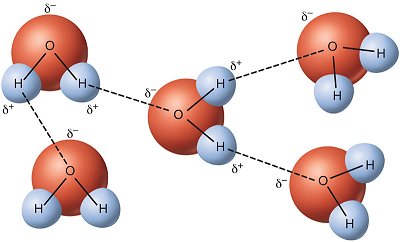
Cohesion is the attraction of water molecules to each other, and adhesion is the attraction of water to other polar substances. In addition to cohesion and adhesion, other special properties of water due to hydrogen bonding include water’s
- high heat capacity
- high heat of vaporization
- ability to dissolve numerous polar molecules
Understanding these characteristics, and more, helps us understand and appreciate water’s extreme importance in maintaining life on Earth and will be discussed below. (It is important to note that even though we are only focusing on water in this chapter, hydrogen bonding also occurs in other substances that have polar molecules.)
The 3 States of Water on Earth
Water on Earth can naturally exist as either solid, liquid, or gas depending on the prevailing temperature and pressure conditions. In liquid water, hydrogen bonds are constantly being formed and broken as water molecules slide past each other. The energy of the moving water molecules (kinetic energy) is responsible for breaking the bonds. When heat is added to water (increasing the temperature), the kinetic energy of the molecules goes up and more bonds are broken. As more heat is added to boiling water, the higher kinetic energy of the water molecules causes the hydrogen bonds to break completely and allows them to escape into the air as water vapor.
On the other hand, when the temperature of water is reduced and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding (since there isn’t enough energy to break the hydrogen bonds). The crystalline structure, ice, has a more open structure than the liquid form of water. The open structure of ice makes ice less dense than liquid water, a phenomenon not seen in the solidification of other liquids. The lower density of ice causes it to float at the surface of liquid water, such as an iceberg in the ocean or ice cubes in a glass of ice water.
Video 14.1.1. Excellent explanation why ice floats in water. (3:56)
In lakes and ponds, ice will form on the surface of water creating an insulating barrier that protects animals and plants that live in the water from freezing. Without this layer of insulating ice, they would freeze in the solid block of ice that would form, and they would not survive. The ice crystals that form upon freezing would rupture the delicate membranes essential for the function of living cells, irreversibly damaging them.
Cohesion and Adhesion
As shown in Figure 14.1.1 above, cohesion results in water molecules being attracted to other water molecules. This is what allows drops of water to mound up amazingly high on a penny before spilling over the edge.

Adhesion, or the attraction between water molecules and other molecules, is sometimes stronger than water’s cohesive forces, especially when water is exposed to charged surfaces such as on the inside of thin glass tubes known as capillary tubes. Adhesion is observed when water “climbs” up the tube placed in a glass of water. Notice in Figure 14.1.2 below that the water appears to be higher on the inner sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary tube more than they are to each other and, therefore, adhere, or stick, to it. This type of adhesion is called capillary action. You may have had experience with this when using a graduated cylinder to measure a volume of water, as well. Viewed from the side, the level of water is not flat. The proper measurement will be read on the scale at the level of the bottom of the curved surface, or meniscus.
Adhesion is also involved in the movement of water and nutrients from the soil around the root systems to other parts of plants above the ground. Related to this, it also plays a role in moving water from the plants to the atmosphere via transpiration as part of the water, or hydrologic, cycle.
Cohesion is also involved in capillary action. Water molecules adhering to the walls of a charged tube are also attracted to other water molecules, helping pull other water molecules up above the original surface of the water.
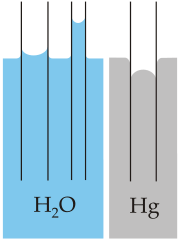
With the tubes in mercury rather than glass, cohesion in mercury is stronger than its adhesion to the class so the curved surface of the liquid is opposite what it was with water.
Water as the Universal Solvent
Because of water’s polarity, ions and polar molecules can readily dissolve in it. Water is, therefore, referred to as a solvent. Water is capable of dissociating, or dissolving, more substances (polar substances) than any other liquid, earning it the title of “universal solvent.”
How does this occur? The charges associated with other polar molecules and ions allow these particles to form hydrogen bonds with water, surrounding the particle with water molecules. Recall that in each water molecule, oxygen has a slightly negative charge, while the two hydrogens have a slightly positive charge. The slightly negative particles of a compound will be attracted to water’s hydrogen atoms, while the slightly positive particles will be attracted to water’s oxygen atom. This causes the compound to dissociate. Think of this as water molecules essentially pulling apart another polar substance.
Video 14.1.2. Good illustration of how water dissolves ionic substances. (2:23)
This process is very important as it enables water to dissolve various chemicals and distribute them within living organisms, including removing toxic substances from living things. In the environment. it is able to dissolve or dissociate many types of particles into products necessary in both biotic (living) and abiotic (nonliving) processes.
Water itself dissociates into ions in acid-base reactions. This involves pH, which measures acidity level, and is important in many important human and environmental chemical reactions.
High Heat Capacity
Water has the highest specific heat capacity of any liquid. Water’s high heat capacity is another property caused by hydrogen bonding among the water molecules. Specific heat is defined as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie. It takes water a long time to heat up and a long time to cool down. In fact, the specific heat capacity of water is about five times more than that of sand. This explains why land cools faster than the sea.
Due to its high heat capacity, warm blooded animals use water to disperse heat more evenly and maintain temperature in their bodies: it acts in a similar manner to a car’s cooling system, transporting heat from warm places to cool places, causing the body to maintain a more even temperature. This is just one of many reasons that hydration is so important to our bodies.
Heat of Vaporization
Water also has a high heat of vaporization, which is the amount of energy required to change one gram of a liquid substance to a gas. A considerable amount of heat energy (586 calories) is required to accomplish this change in water. This process occurs on the surface of water. As liquid water heats up, hydrogen bonding makes it difficult to separate the liquid water molecules from each other, which is required for it to enter the gas phase (steam). Thus, water acts as a heat sink and requires much more heat to boil than liquids such as ethanol, whose hydrogen bonds are weaker. Eventually, as water reaches its boiling point of 100° Celsius (212° Fahrenheit), the heat can break the hydrogen bonds between the water molecules, and the kinetic energy between the water molecules allows them to escape from the liquid as a gas.
Even when below its boiling point, water’s individual molecules acquire enough energy from other water molecules such that some surface water molecules can escape and vaporize. This process is known as evaporation. Since hydrogen bonds need to be broken for water to evaporate means that a substantial amount of energy is used in the evaporation process. As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation is taking place. In many living organisms, including in humans, the evaporation of sweat, which is 90 percent water, allows the organism to cool so that homeostasis of body temperature can be maintained.
Summary
Water’s polarity and resulting hydrogen bonding contribute to the following critical properties of water:
1. Water exists in nature as a solid, liquid, and gas, with ice (the solid phase of water) less dense than liquid water.
2. Water is cohesive, displaying high surface tension.
3. Water is adhesive, capable of capillary action.
4. Water is a universal solvent.
5. Water has high heat capacity.
6. Water has a high heat of vaporization.
A substance with its atoms arranged in a solid, repetitive structure.

