9.4 Mining Techniques
The style of mining is a function of available technology, social license, and economics. It is in the best interest of the company extracting the resources to do so in a cost-effective way. [3] But profit for the company does not always align with what is best for the environment. Metal deposits are mined in a variety of different ways, depending on their depth, shape, size, and grade. Relatively large deposits that are quite close to the surface and somewhat regular in shape are mined using open-pit mine methods, creating giant holes in the ground, such as the one shown on the Bisbee Lavender Pit. This method is cheaper than making an underground mine, but it is also less precise, so it is necessary to mine a lot of waste rock along with the ore.
Video 9.4.1. Flight over the Lavender Pit copper mine, Bisbee, Arizona. The colors in the rock walls are typical for copper ores. By S. Dorsey (3:02).
Underground mining is often used for deposits with high ore-mineral content, more localized, or very concentrated resources.
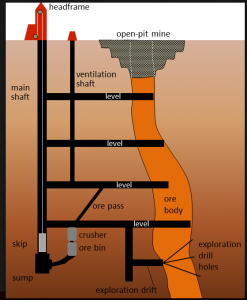
Some ore minerals are mined underground by introducing chemical agents that dissolve the target mineral followed by solution extraction and subsequent precipitation in a surface operation, but more often a mining shaft/tunnel (or a large network of these shafts and tunnels) is dug to access the material (Fig 9.4.1). In this way, it is possible to focus the mining on the ore body itself. However, with relatively large ore bodies, it may be necessary to leave some pillars to hold up the roof [1].
Mineral Processing
Video 9.4.2. How do we separate the economic mineral from the rest of the rock? Watch this short video to visualize what a mineral processor does (1:23).
All ore minerals are mixed with less desirable components called gangue. The process of physically separating gangue minerals from ore-bearing minerals is called concentrating. Separating a desired element from a host mineral by chemical means (including heating in the presence of other minerals) is called smelting. Finally, taking a metal such as copper and removing other trace metals such as gold or silver is done through the process of refining.
Typically, this is done one of three ways:
1. Items can either be mechanically separated and processed based on the unique physical properties of the ore mineral, like recovering placer gold based on its high density;
2. items can also be heated to chemically separate desired comconcentratingponents, like refining crude oil into gasoline; or
3. items can be smelted, in which controlled chemical reactions unbind metals from the minerals they are contained in, such as when copper is taken out of chalcopyrite (CuFeS2).
Mining, concentrating, smelting, and refining processes require enormous amounts of energy. Continual advances in metallurgy and mining practice aim to develop ever more energy-efficient and environmentally benign processes and practices. [1]
Video 9.4.3. This video shows walks you through all the different stages involved in separating copper from the rock and concentrating it in a step-wise fashion (7:40).
As you can see from the video, you need large amounts of water to process the metal. Mining operations need to have plans in place to dispose of the water in more or less suitable conditions.
Exercises. How much copper do you need for a car?
To get copper for a car, you need to mine one or more copper-bearing minerals, for example, malachite, azurite, or bornite, among others. Each mineral has a different copper concentration. Let’s find out how much copper could be extracted from bornite.
- Bornite has a chemical formula of Cu5FeS4. From the formula, we can see that it has 5 atoms of copper, 1 atom of iron, and 4 atoms of sulfur.
- If we multiply the number of atoms of the element by the atomic weight we can calculate the total weight of the element in the mineral.
|
Element |
Number of Atoms | Atomic Weight | Total Weight (AMU) |
| Cu | 5 | 64 | 320 |
| Fe | 1 | 56 | 56 |
| S | 4 | 32 | 128 |
| Molecular Weight | 504 | ||
- We can find the molecule weight by adding all the total weights, in this case, 504 AMU (atomic mass units)
- To find the fraction of copper in bornite, divide the total weight of copper in the mineral, 320, by the molecule weight, 504.
320/504 = 0.63; 0.63 *100 = 63% of copper per molecule of bornite.
Try this exercise with azurite Cu3 (CO3)2 (OH)2 and malachite Cu2 (CO3) (OH)2
Which mineral has more copper? Which one is less profitable?
Valuable material in the Earth, typically used for metallic mineral resources.
Material found around ore which is less valuable and needs to be removed in order to obtain ore.
Removing trace elements from the desired element
gold found on river beds
A mechanical process which takes ore and separates it from gangue material.

