10.1 Fossil Fuels – An Overview
Jeff Simpson and Carolina Londono Michel
Learning Objectives – By the end of this chapter, you should be able to do the following.
- Describe the similarities and differences in formation of the three fossil fuels.
- Explain why fossil fuels are called hydrocarbons.
- Link the energy in a molecule of a fossil fuel to the energy from the sun.
- Explain why fossil fuels have been our preferred energy source.
The term fossil fuel describes a group of energy sources that were formed from ancient plants and animals during the Carboniferous period approximately 362-286 million years ago. This was long before the appearance of the dinosaurs ~252 million years ago.
Video 10.1.1 – Fossil Fuels (1:01)
Important Points From this Video
- Fossil fuels formed in the Carboniferous Period, 360-286 MYA or million years ago. (This can vary from as long ago as 541 MYA to – 65 MYA.)
- Three variables in creating a fossil fuel are time, temperature and the starting organic matter.
- Coal comes from ferns, plants and trees.
- Oil comes from small organisms like zooplankton and algae where conditions caused complex organic material to reform.
- (Natural gas) starts the same as oil but undergoes more heat and pressure.
- Fossil fuel are desirable as they have high energy density, a lot of energy in a small mass.
- Fossil fuels are used for electricity & transportation. (They also are used in industry for heat.)
- The unequal distribution of fossil fuels leads to global conflict.
- Many of the easy-to-obtain fossil fuel deposits have been extracted.
At that warm time in Earth’s history, unlike today’s world with high mountains and deep oceans, much of Earth’s warm surface was covered with swamps and shallow seas teeming with microscopic animals and plants. As those organisms died, they sank to the bottom of those swamps and oceans, and, over millions of years began to break down under layers of sand, clay, and other minerals, leaving behind mostly carbon and oxygen. Just as you cooking in your kitchen, nature starts with different ingredients (depending on the organic material present) and applies pressure, heat, and time. Depending on nature’s ‘baking’ process, the starting products can be transformed into either coal, petroleum (oil), and/or natural gas (natural gas).
Natural gas was formed by the same process as oil but was exposed to more heat and pressure, causing it to further decompose and turn into a gaseous form.
Video 10.1.2 – Formation of Fossil Fuels (2:26)
Important Points From this Video
- Oil, coal and natural gas are made over hundreds of millions of years from tiny plants and animals that died and were buried on oxygen-poor water environments.
- The energy in the fossil fuels started by bonds formed from sunlight. Coal comes from ferns, plants and trees.
- Woody plants result in coal, slimy plants like algae produce oil, and both can result in natural gas.
- Burning these fossil fuels is reversing the process that stored all this carbon in Earth’s crust for hundreds of millions of years.
Fossil fuel resources have powered industrialization. Fossil fuels have a variety of applications from electricity production to transport fuels. They can also be used to make a variety of common products from plastics to cosmetics to even some medicines.Because they were at one time plentiful, easy to burn, and energy dense, they became the world’s dominant energy source.
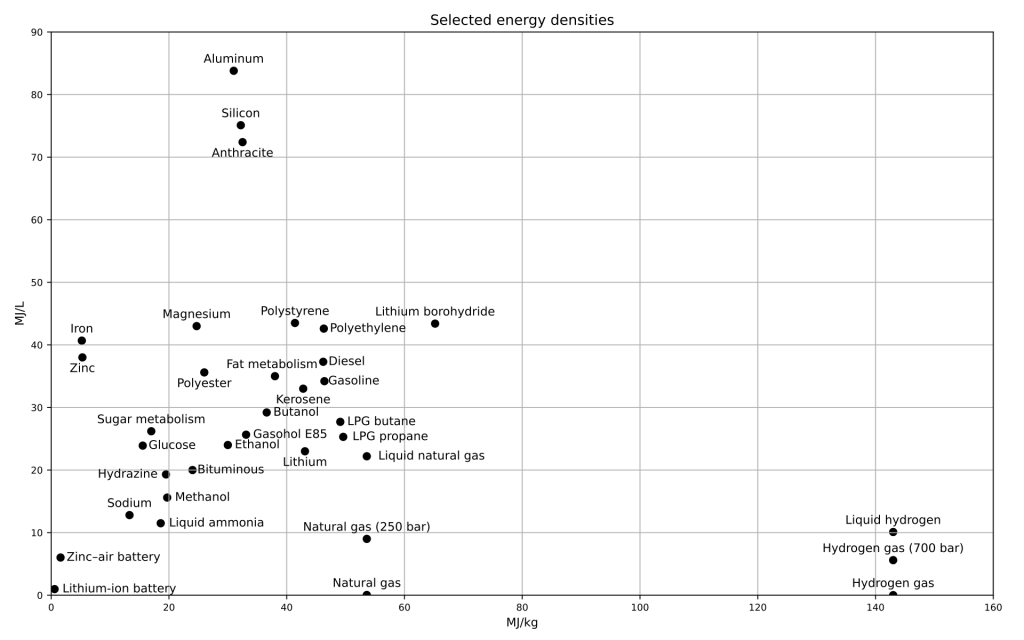
Fossil fuels can be an abundant and cheap or in some cases a scarce and expensive form of energy depending on geographic location. For this reason, geopolitical issues arise due to scarcity caused by the varied natural geographic allocation of these valuable resources.
Fossil fuels are considered nonrenewable resources because they take millions of years to form which means that once they are used, the resources will not be replenished in a human lifetime.The gradual depletion of the most accessible fossil fuel reserves has forced companies to develop technologies for extracting more challenging or unconventional reserves. In many cases, this means additional safety and environmental concerns as well as higher costs. Fossil fuels are also the largest emitters of carbon dioxide, a greenhouse gas which causes climate change. In addition, their production causes both environmental and human health issues. These concerns are leading to to develop alternate sources of energy that are more environmentally sustainable and renewable.
Hydrocarbons
Fossil fuels are extractable sources of stored energy created by ancient ecosystems. The natural resources that typically fall under this category are coal, oil (petroleum), and natural gas. Living organisms such as plants, phytoplankton, algae, and cyanobacteria captured sunlight energy via photosynthesis. We could refer to these resources as fossil solar energy since the energy of the sun in the past has been converted into chemical energy within a fossil fuel. Fossil fuels are molecular chains of hydrogen and carbon, thus the name Hydrocarbons. Of course, as the energy is used, the once stored carbon enters the atmosphere, causing consequences for our climate system. Fossil fuels account for much of the energy used in the world [1].
Transforming matter from living organisms into fossil fuel hydrocarbons is complex. Petroleum did not formed from dinosaur bones. Much oil did form during the dinosaur era, the Mesozoic Era, but T-Rex and the megafauna contributed little to it. The real energy heroes are the plants and marine algae – plankton – that lived at that time. But why? How?
During much of the Mesozoic, the earth’s climate was tropical, warm, and without polar ice caps. It was a plankton paradise with room to live and plenty of sunshine to create new molecular bonds. The continents were in different positions, maximizing the ocean surface where the algae lived and changing the way the ocean currents flowed. In fact, there was not much movement at the bottom of the ocean during the Mesozoic. (Fig 10.3). Without mixing and vigorous circulation, the oxygen did not reach the ocean floor and vast areas of it were low in oxygen or anoxic. These conditions facilitated the chemical reactions needed to form hydrocarbons. The low oxygen conditions also prevailed in swamps, bogs, marshes which were common during this Paleozoic (the era before the Mesozoic). In fact, most of our coal resources were deposited during the geologic period Carboniferous (part of the Paleozoic era); Carboniferous means coal-bearing, indicating the widespread deposition and formation of coal.

Key Takeaways
- Fossil fuels and biofuels are organic matter that formed from the sun’s energy millions of years ago.
- Most of a fossil fuel is made of the element carbon.
Cooking Fossil Organic Matter in Earth’s Kitchen
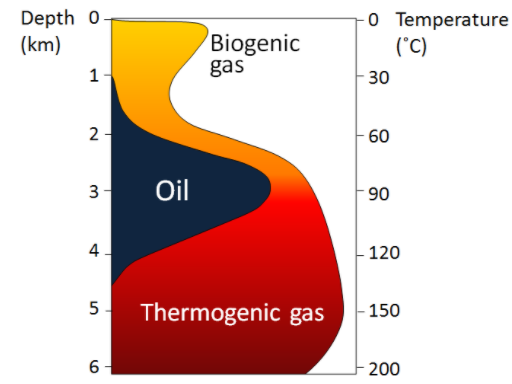
How does Earth turn organic matter into hydrocarbons? First, plants and plankton die and fall to the bottom of the swamp or sea, respectively, where they are buried under oxygen-poor sediments. They do not decompose as decomposition needs oxygen but rather reduced. (Rapid burial is the key). The chemical energy within the organisms’ tissues is transferred to surrounding geologic material (sediments, minerals). Again, the key here is that organisms DO NOT decompose but instead transform into something else under the right heat and pressure conditions. It takes a defined range of temperature and pressure conditions over defined amounts of time to produce hydrocarbons, “the oil generation window“! Heat and pressure applied after the burial of sediments also can transform the organic matter trapped in them into “higher quality” materials (brown coal to anthracite, oil to gas) and/or migration of mobile materials, such as gas.
An Abbreviated History of Fossil Fuels
Video 10.1.3 – 300 Years of Fossil Fuels (5:38)
Main Points from the Video
1. We need to learn to live without burning fossil fuels.
2. We need to adapt to the end of economic growth as we have known it.
3. We need to support a population of 8,000,000+ people and growing.
4. We have to deal with our legacy of environmental destruction.
A type of fuel source that has formed on the planet from natural processes.
small animals that drift in the ocean
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Also present usually are low levels of trace gases like carbon dioxide, nitrogen, hydrogen sulfide, and helium.
An organic sedimentary rock formed by the compaction of plant and animal organic matter over millions of years.
Petroleum is a yellowish-black liquid found in geological formations. It commonly is refined into various fuels and chemicals. The components of petroleum are separated by means of distillation. Petroleum mainly consists of hydrocarbons as well as traces of other organic compounds. The term petroleum covers both naturally occurring unprocessed crude oil and petroleum products that are made up of refined crude oil. A fossil fuel, petroleum is formed when large quantities of dead organisms, mostly zooplankton and algae, are buried underneath sedimentary rock and subjected to both prolonged heat and pressure.
Fuel formed from biomass by a
process taking millions of years or longer.
A major subdivision of geologic time from 251 Ma to 65 Ma; characterized by dinosaurs.
Without oxygen

